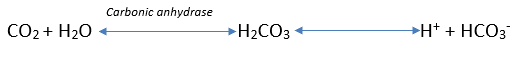Carbon dioxide (CO2) is the major waste product of aerobic respiration. Too much or too little CO2 in the blood can lead to serious consequences. This article will consider CO2 transport in the blood, its role in maintaining blood pH and conclude by discussing its clinical relevance.
Carbon Dioxide in the Blood
The main role of CO2 is to regulate the pH of the blood – this is much more important that transporting CO2 to the lungs for exhalation.
Figure 1 shows how CO2 dissolves in the blood. The conversion of carbonic acid (H2CO3) to a hydrogen and bicarbonate ion (H+ + HCO3–) is almost instantaneous. A small amount of dissolved CO2 produces a small rise in hydrogen ions which is capable of altering the blood pH. The proportion of CO2 to HCO3– is critical and explains why this occurs.
This ratio is roughly 1:20, therefore a rise of 1 CO2 requires a corresponding rise of 20 HCO3– to prevent alterations to blood pH by buffering the increase in acidity. As detailed in the reaction above only 1 HCO3–, is generated from every CO2 therefore the blood pH will become more acidic due to the excess hydrogen ions. There must therefore be an alternate method of transportation to prevent severe acidosis every time we respire and create CO2.
Methods of Transport
CO2 is transported in the blood in 3 ways:
- Carbamino Compounds
- Hydrogen Carbonate (HCO3–)
- Dissolved CO2
Carbamino Compounds
About 30% of all CO2 is transported as carbamino compounds. At high concentrations carbon dioxide directly binds to amino acids and the amine groups of haemoglobin to create carbaminohaemoglobin. Carbamino formation is most effective at the periphery where CO2 production is high due to cellular respiration.
The Haldane effect also contributes to the formation of carbamino compounds. This occurs where O2 concentration is lower (as in the active peripheries where O2 is being consumed) and causes the CO2 carrying capacity of the blood to increase. This is because release of O2 from Hb promotes binding of CO2.
Formation of carbamino compounds achieves 2 goals:
- Stabilising pH – CO2 is unable to leave the blood cell to contribute to changes in pH
- Bohr effect – it stabilises the T state of haemoglobin, promoting the release of O2 from the other subunits of haemoglobin into the tissues that are most active, undergoing the most respiration and producing the most CO2
When the blood cell reaches areas of high O2 concentrations again (such as the lungs), it preferentially binds O2. This stabilises the R state, which promotes the release of CO2 (Haldane effect). This allows more O2 to be picked up and transported in the blood.
HCO3– ions
60% of all CO2 is transported through production of HCO3– ions in the red blood cell. This is explained in the diagram below (Figure 2). CO2 diffuses into the red blood cells and is converted to H+ and HCO3– by an enzyme called carbonic anhydrase. This HCO3– is transported back into the blood via a chloride-bicarbonate exchanger (aka anion exchanger/AE). The HCO3– can now act as a buffer against any hydrogen in the blood plasma.
The H+ created by the carbonic anhydrase reaction in the red blood cell binds to haemoglobin to produce deoxyhaemoglobin. This contributes to the Bohr effect as O2 release from haemoglobin is promoted in active tissues where H+ concentration is higher. It also prevents hydrogen entering the blood to lower pH, stabilising the pH.
When the red blood cells reach the lungs, oxygen binds to the haemoglobin and promotes the R state, allowing the release of H+ ions. These hydrogen ions become free to react with bicarbonate ions to produce CO2 and H2O, where the CO2 is exhaled. Thus the high O2 concentrations reduce the CO2 carrying capacity of blood, in accordance with the Haldane effect.
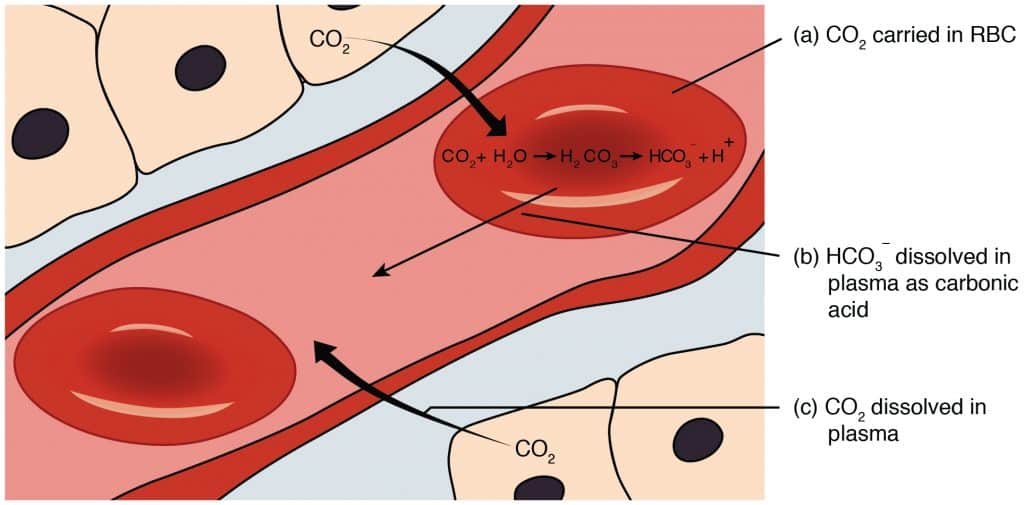
Fig 2 – Diagram showing methods of transporting carbon dioxide in the blood. The reaction producing bicarbonate is shown within the red blood cell.
Dissolved in Plasma
About 10% of all CO2 is transported dissolved in plasma. The amount of gas dissolved in a liquid depends on its solubility and the partial pressure of the gas. CO2 is very soluble in water (23x more soluble than O2) and the partial pressure of inspired CO2 is ~40mmHg. Despite its solubility, only a minority of the total CO2 in blood is actually transported dissolved in plasma.
The partial pressure is higher in the periphery where tissues are producing CO2 and lower at the alveoli where CO2 is being released. This allows more CO2 to be dissolved in the periphery whilst at the alveoli it is released into the gas phase as the partial pressures are lower.
Clinical Relevance – Metabolic Acidosis
Acidosis occurs when the pH of the blood falls below 7.35. It can be broadly classified as metabolic or respiratory acidosis.
Metabolic acidosis can result from an excess of H+ production or a reduction in the HCO3– buffer. Conditions such as diabetic ketoacidosis can increase acid production. However, a disorder of the kidneys themselves such as chronic kidney disease may reduce HCO3– production.
In such cases, the respiratory system attempts to compensate by increasing respiration rate (hyperventilating). This allows one to “blow off” some CO2 reducing the acidity of the blood. However, the main correction must be accomplished by the kidneys which can both increase hydrogen excretion to reduce the acidity of the blood and increase bicarbonate reabsorption to allow increased buffering of blood acidity.
Symptoms of acidosis include rapid breathing (to blow off CO2), confusion, fatigue and headache. It is important to identify it as it can be mistaken for intoxication.