Oxygen is transported to various tissues in the body via the bloodstream. This article will discuss the process of oxygen transport, factors that affect it and consider its clinical relevance.
Transport of Oxygen
Oxygen is transported in the blood in two ways:
- Dissolved in the blood (1.5%)
- Bound to haemoglobin (98.5%)
Bound to Haemoglobin
Once oxygen has entered the blood from the lungs, it can be bound by haemoglobin (Hb) in the red blood cells.
Haemoglobin is a protein comprised of four subunits: two alpha subunits and two beta subunits. Each subunit has a haem group in the centre that contains iron and binds one oxygen molecule. This means each haemoglobin molecule can bind four oxygen molecules, forming oxyhaemoglobin. Haemoglobin molecules with a greater number of oxygen molecules bound are brighter red. This is why oxygenated arterial blood is brighter red and deoxygenated venous blood is darker red.

Fig 1 – Haemoglobin molecule. Note the four haem groups in green
Oxygen Binding to Haemoglobin
Haemoglobin changes shape based on the number of oxygen molecules bound to it. The change in shape also alters its affinity to oxygen. As the number of oxygen molecules bound to haemoglobin increases, the affinity of haemoglobin for oxygen increases. This is known as cooperativity.
When no oxygen is bound, the haemoglobin is said to be in the Tense State (T-state), with a low affinity for oxygen. At the point where oxygen first binds, the haemoglobin alters its shape into the Relaxed State (R-state), which has a higher affinity for oxygen. We can plot this change on a graph of oxygen saturation over partial pressure of oxygen.
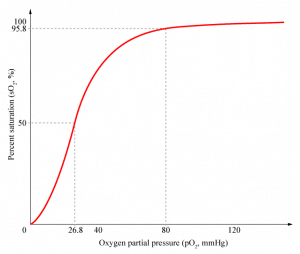
Fig 2 – Saturation of Hb with oxygen against partial pressure of oxygen
Oxygen Delivery at Tissues
As shown on the diagram above, the percentage of oxygen bound to haemoglobin is related to the partial pressure of oxygen (pO2) at a given site. When oxyhaemoglobin reaches a tissue with a low pO2 (e.g. skeletal muscle), it will dissociate into oxygen and haemoglobin, resulting in an increase in local pO2. When it reaches a tissue that has a high pO2 (e.g. in the pulmonary circulation), haemoglobin will continue to take up more oxygen, resulting in a lowered pO2.
Factors Affecting Oxygen Affinity
Various factors can affect the affinity of haemoglobin for oxygen:
- pH/pCO2 – When H+/pCO2 increases and pH decreases, Hb enters the T state and its affinity for oxygen decreases. This is known as the Bohr effect. Inversely, when H+/pCO2 decreases and pH increases, the affinity of haemoglobin for oxygen increases.
- 2,3-diphosphoglycerate (2,3-DPG) – 2,3-DPG (sometimes referred to as 2,3-BPG) is a chemical found in red blood cells. It is a product from the glucose metabolic pathway. 2,3-DPG binds to the beta chains of haemoglobin, so increased 2,3-DPG levels results in it binding to haemoglobin, decreasing the affinity of haemoglobin for oxygen. Conversely, when there are decreased 2,3-DPG levels, e.g states of decreased tissue metabolism, there are fewer 2,3-DPG molecules to bind to haemoglobin. This means there are more opportunities for it to bind and therefore it has a higher affinity for oxygen.
- Temperature – At increased temperatures, for example in active muscles, there is an increase in heat production which decreases the affinity of haemoglobin for oxygen. At decreased temperatures, e.g. decreased metabolic states, the reduced heat production means the affinity of haemoglobin for oxygen increases.
The affinity of haemoglobin for oxygen also results in a shift in the oxygen-haemoglobin dissociation curve. An increase in oxygen affinity results in the curve shifting to the left, whereas a decrease in oxygen affinity results in the curve shifting to the right.
Clinical Relevance – Carbon Monoxide Poisoning
Carbon Monoxide (CO) is a colourless, odourless gas. It can be released from faulty boilers or combustion engines. Carbon Monoxide poisoning occurs when CO reacts with haemoglobin at the site of oxygen binding. Haemoglobin has an affinity for CO that is 210x greater than its affinity for oxygen. This means that once carbon monoxide binds to haemoglobin, it is irreversible.
Symptoms of CO poisoning are headache, nausea and tiredness. Interestingly, respiration rate is usually spared as the partial pressure of oxygen dissolved in the blood is maintained at normal levels. Haemoglobin bound to CO has a cherry-red colour and this may be visible in nails beds and mucous membranes of patients with CO poisoning. Treatment is with 100% oxygen and referral for hyperbaric oxygen treatment. CO poisoning is considered fatal when 70-80% of haemoglobin is bound with carbon monoxide.
