Like skeletal muscle, cardiac myocytes contract according to the sliding filament theory of muscle contraction.
In this article, we will look at the process of calcium-induced calcium release and the electrical coupling of cardiac myocytes.
Calcium-Induced Calcium Release
Calcium can be released from the sarcoplasmic reticulum (SR) in 2 ways:
- Via IP3
- Gq-protein coupling facilitates the release of Ca2+ from the sarcoplasmic reticulum. This is particularly important in muscle types that require calcium-induced calcium release (e.g. in cardiac muscle).
- Gq activates the effector: Phospholipase C enzyme
- Phospholipase C breaks down PIP2 (phosphatidylinositol 4,5-biphosphate), into IP3 + DAG
- IP3 then binds to the IP3 receptor on the SR
- DAG acts on various proteins (e.g PKC)
- Ca2+ channels on the SR open and Ca2+ is released.
- Action is then terminated by IP3 phosphatase (IP3 is cleaved to IP2)
- Via Ryanodine receptors (RyR): these are a family of Ca2+ releasing channels found on intracellular organelles that store or release Ca2+.
- Membrane depolarisation opens voltage-operated calcium channels (VOCCs) in the T-tubule system and calcium is released.
- Calcium binds to RyR on the sarcoplasmic reticulum. This induces conformational changes in a Ca2+ channel which is closely associated with RyR.
- RyR is activated and opens to release Ca2+ from the SR stores. This is known as the ‘calcium spark’.
Released calcium leads to a calcium spike. Calcium binds to troponin C thus activating the cross-bridge cycling mechanism for contraction.
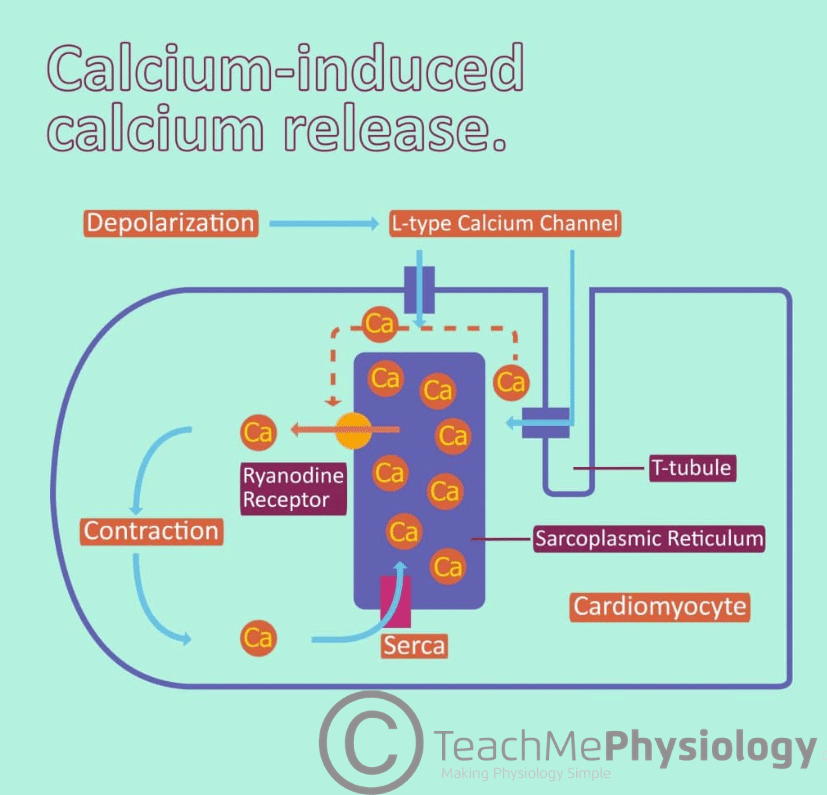
Fig 1 – Diagram showing the process of Calcium Induced Calcium Release (CICR).
Pathway of Contraction
Pacemaker cells in the SA and AV nodes initiate an action potential which is conducted around the heart via gap junctions.
The action potential travels down the T-tubules between sarcomeres resulting in an influx of calcium ions into the sarcoplasm through VOCCs.
When calcium enters the sarcoplasm (through VOCCs and ryanodine receptors) it binds to cardiac troponin-C which moves the tropomyosin away from the actin-binding site thus exposing it and initiating cross-bridge binding.
Sliding Filament Model of Contraction
Cardiac muscle contraction occurs via the sliding filament model of contraction, much like skeletal muscle. Once calcium is bound to troponin-C and the conformational change of tropomyosin has occurred, myosin heads can bind to actin.
Following this ADP and inorganic phosphate are released from the myosin head so the power stroke can occur. In this the myosin head pivots and bends, pulling on the actin and moving it, causing muscle contraction.
After this occurs a new molecule of ATP binds to the myosin head, causing it to detach from the actin. Finally, the ATP is hydrolysed into ADP and inorganic phosphate. Following this, the cycle can begin again and further contraction can occur.
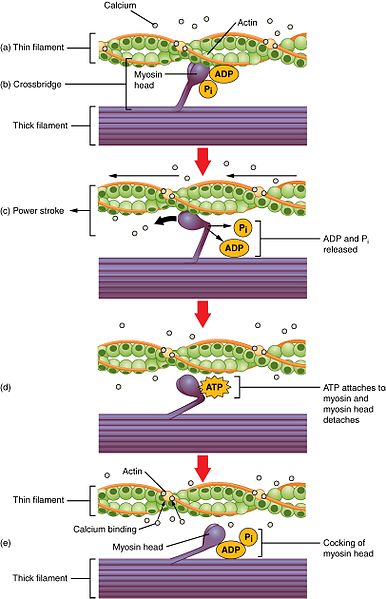
Fig 2 – Diagram showing the sliding filament model of muscle contraction.
Removal of Calcium
After the stimulus is removed, intracellular calcium is then reduced in two ways:
- By entering the sarcoplasmic reticulum for storage via a SERCA (sarco(endo)plasmic reticulum calcium-ATPase) channel at the expense of an ATP molecule.
- Through an NCX (sodium-calcium exchange) channel which extrudes a calcium ion and admits a sodium ion when membrane repolarisation starts.
Calcium is no longer bound to troponin C and the actin-binding site is covered up, ending contraction and relaxing the muscle.
Clinical Relevance – Troponin Assays
Some subtypes of troponin can be used as very specific and sensitive markers of damage to the cardiac muscle, particularly troponin I and T. They are typically measured in the blood to differentiate between unstable angina and myocardial infarctions, as they may present with similar symptoms.
The cardiac troponin levels in a patient suffering from a myocardial infarction would typically be hugely elevated. These elevated troponin levels would not be seen in unstable angina. Depending on the onset of the myocardial infarction, troponin levels peak a few hours following injury and can remain high for up to two weeks after the event.
However, it is important to note that troponin release is not specific to myocardial infarction and only indicates cardiac muscle damage. As such, they may also be elevated in conditions such as heart failure, pericarditis, and amyloidosis. In addition to this, non-cardiac diseases such as sepsis or renal failure can also elevate troponins.
