Potassium (K+) is a hugely important electrolyte within the body and plays a vital role in maintaining the resting membrane potential of cells. Even a small change in the extracellular concentration of K+ could significantly depolarize or hyperpolarize cells, which has implications for cardiac function, amongst other things.
The concept of maintaining an internal balance of K+ refers to its movement between the extracellular fluid (ECF) and intracellular fluid (ICF). The normal concentrations of K+ are between 3.5-5.0 mmol/L in the ECF and between 120-150 mmol/L intracellularly. 98% of the body’s K+ is stored intracellularly.
Mechanisms for Shifting Potassium Into Cells
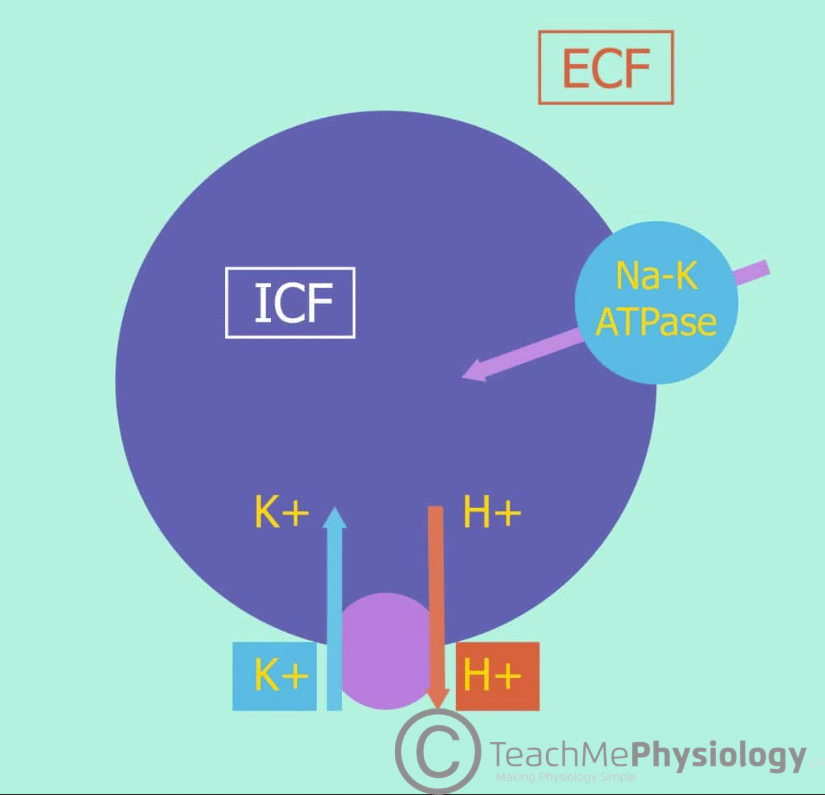
Potassium movement in and out of cells is controlled mainly by Na+/K+ ATPase. This is a transmembrane pump which uses active transport to move 3 Na+ ions out of the cell (extracellularly) and 2 K+ ions into the cell (intracellularly). It maintains ion gradients by moving ions against their concentration gradients, as Na+ has a higher concentration extracellularly and K+ has a higher concentration intracellularly.
An increase in [K+] in the ECF will promote the movement of K+ into cells, down its concentration gradient. However, the internal balance of K+ is not this simple and there are other factors to consider.
When we eat a meal, insulin is secreted by the pancreas in response to increased K+ concentrations in the blood. This increases the activity of Na+/K+-ATPase, therefore moving the excess K+ into cells. This homeostatic mechanism helps to prevent dangerous rises in [K+] after eating. Other hormones which act on Na+/K+-ATPase channels include aldosterone and catecholamines.
Alkalosis will also cause a decrease in ECF [K+]. This is due to the close relationship of [K+] with the pH of the ECF. A decrease in the concentration of H+ ions (i.e. an increase in pH) will lead to H+ ions being transported out of cells. This causes a reciprocal shift of K+ into cells, thereby lowering the concentration of K+ in the ECF.
Conversely, an increase in H+ ion concentration (acidosis) causes H+ to move into cells, drawing K+ out.
Mechanisms for Shifting Potassium Out of Cells
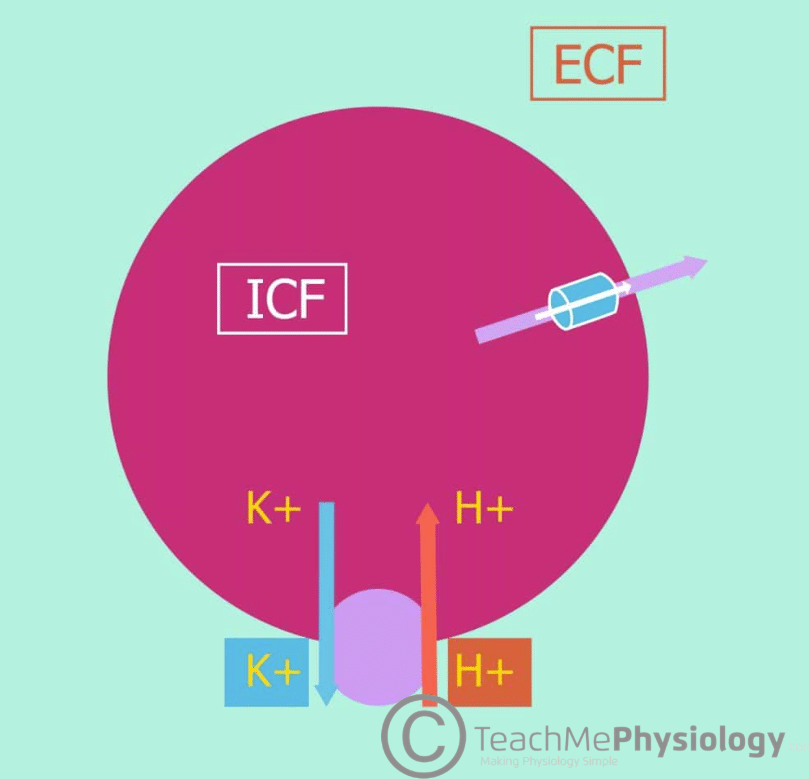
A low concentration of ECF K+ promotes the movement of K+ out of cells, down its concentration gradient.
During exercise, skeletal muscle contracts, causing a net release of K+ from these cells. This leads to an increase in ECF [K+] proportional to exercise intensity. Hyperkalaemia is prevented due to the uptake of K+ by other non-contracting cells.
Furthermore, exercise causes the release of catecholamines e.g. adrenaline which increases K+ uptake by other cells via Na+/K+-ATPase channels as mentioned earlier. This can lead to short-term hypokalaemia when exercise is stopped.
Acidosis also causes an increase in the concentration of ECF K+. Cell lysis will also cause the release of intracellular K+ into the ECF. If plasma tonicity becomes increased, water will leave cells to compensate. This will increase the intracellular concentration of K+, causing it to leave the cell and enter the ECF down its concentration gradient.
Clinical Relevance – Hypokalaemia
Hypokalaemia is defined as extracellular [K+] < 3.5mmol/L. This usually occurs due to a disruption of internal balance or increased loss of potassium, either from the kidneys or from elsewhere. A problem with internal balance is metabolic alkalosis. This is because, when H+ ion concentration in the ECF is low, they are transported out of cells in exchange for K+, leading to hypokalaemia. Excessive loss of potassium occurs through either renal or non-renal losses. Loss of K+ via the kidneys can occur if certain types of diuretics are used or if there is osmotic diuresis as a result of diabetes mellitus. Diarrhoea and vomiting are two examples of non-renal losses of K+.
Clinical Features
- Muscle weakness – changes in resting membrane potential can alter the excitability of skeletal muscle. This can make it more difficult for muscles to contract, leading to muscle weakness.
- Paralytic ileus – gut smooth muscle can also be affected by changes in resting membrane potential, potentially causing paralytic ileus. This is where gut peristalsis is slowed or stopped completely, such that bowel obstruction can occur.
- Nephrogenic diabetes insipidus – hypokalaemia may also cause kidneys to become unresponsive to antidiuretic hormone (ADH). As a result, nephrons have a reduced ability to reabsorb water.
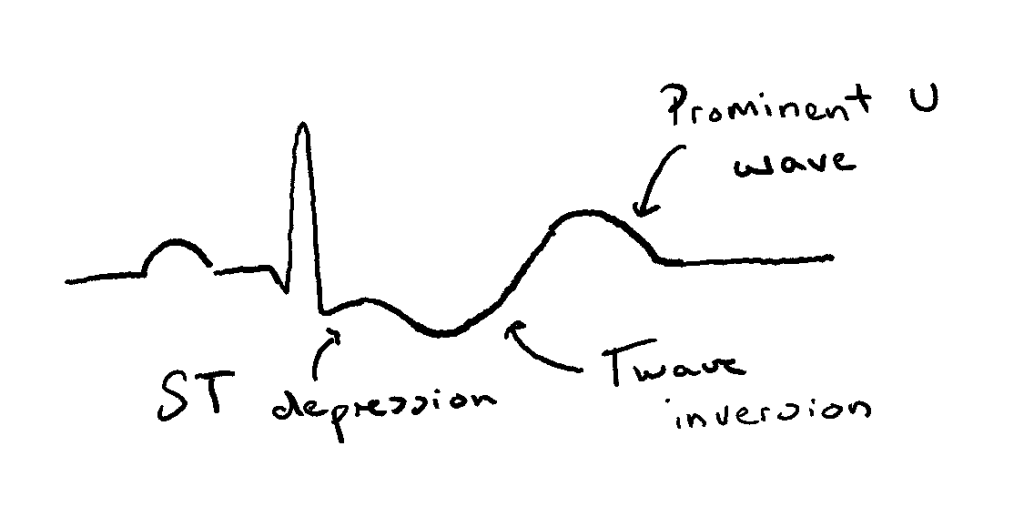
- Cardiac function is also affected due to the chemical gradient of K+ being increased. This causes membrane hyperpolarisation, thus making cardiac cells less excitable, specifically leading to prolonged and abnormal electrical conduction. ECG changes associated with this include:
- Small or inverted T waves
- Prominent U waves
- ST-segment depression (in severe hypokalaemia)
Treatment
Hypokalaemia can be treated through intravenous or oral K+ replacement therapy. However, more specific treatments can vary depending on the cause of the hypokalaemia. For example, if the cause is increased mineralocorticoid activity, K+ sparing diuretics such as amiloride or spironolactone can be used.
Clinical Relevance – Hyperkalaemia
Hyperkalaemia is when there is too much K+ in the ECF outside cells and in the blood. To learn more about it, click here.