T cells/T lymphocytes are white blood cells produced in the thymus gland. They play an important role in adaptive immunity. There are several subtypes of T cells. These include T helper cells, T regulatory cells, T memory cells, and cytotoxic T cells.
These cells have distinct functions and they work together in a complex network involving other immune cells to combat disease. This article will cover the role of the T memory cell and how they help to protect against infection in the long term.
Initial Exposure
Innate T cell Immune Response
When an antigen first enters the body it encounters cells of the innate immune system, e.g. macrophages and dendritic cells. These cells capture and present the antigen (antigen-presenting cells/APCs) and release a variety of cytokines and inflammatory mediators to recruit other immune cells and stimulate the innate immune response.
The antigen-presenting cells then drain into local lymph nodes where they encounter naïve T helper cells and B cells. These initiate the more specialised, adaptive immune response. The antigen exposure causes the naïve T helper cells to differentiate into memory helper T cells. These then proliferate and specialise into Th1 or Th2 roles – leading to the activation of cytotoxic T cells and B cell differentiation.
Adaptive T cell Immune Response
The cytotoxic T cells help with pathogen removal. They recognise and bind to the antigens expressed by pathogens via MHC I molecules. Upon binding to the antigen/pathogen the cytotoxic T cell releases a variety of mediators to destroy the pathogen.
The T helper cells release a variety of cytokines that activate cytotoxic T cells and macrophages and induce B cell differentiation into plasma cells to produce antigen-specific antibodies. These antibodies help to fight the infection by binding to the antigen. Once bound, the antibody prevents the antigen from binding to other targets and also facilitates antigen recognition and removal.
Secondary Exposure
T Memory Cells
These cells serve to ‘remember’ the specific antigen involved in this encounter, so that should this antigen enter the body again the T helper cells would be able to activate B cells much faster. Subsequently, antigen-specific antibodies are produced.
The T helper cells would also stimulate faster expansion of cytotoxic T cells to hasten pathogen clearance from the body. This would lead to a far quicker immune response and faster infection clearance.
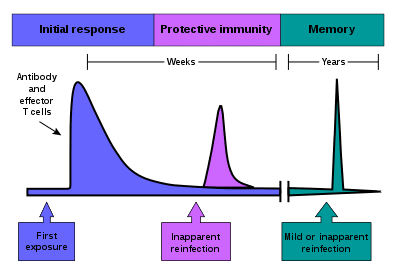
Fig 1 – Graph showing the immune response in the body following vaccination and reinfection – the response is much faster on reinfection due to memory cells in the immune system
Clinical Relevance – T cell Immunodeficiencies
DiGeorge Syndrome
DiGeorge (or 22q11.2 deletion) syndrome is autosomal dominant and caused by a deletion of the q11.2 portion of chromosome 22. The clinical features can vary, but can be remembered with the CATCH-22 acroynm:
- Cardiac abnormalities
- Abnormal facial features
- Thymic hypoplasia
- Cleft palate
- Hypocalcaemia
- Chromosome 22 affected
As T cells usually mature in the thymus after being produced in the bone marrow, thymic hypoplasia causes a reduction in mature, functional T-cells. Most cases are partial, meaning there is only mild to moderate thymus dysfunction which is not life threatening. In complete DiGeorge syndrome, the immunodeficiency can be fatal.
Severe Combined Immunodeficiency
Severe combined immunodeficiency (SCID) is caused by mutations which cause the impaired development of B and T cells. It is the most severe primary immunodeficiency, presenting in early life with severe bacterial, viral and fungal infections. Infants may also present with chronic diarrhoea and failure to thrive.
The two most common causes are X-linked and autosomal recessive adenosine deaminase deficiency.
