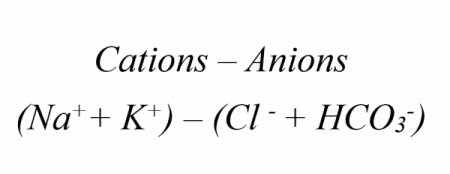The acid-base balance is vital for normal bodily functions. When this equilibrium is disrupted, it can lead to severe symptoms such as arrhythmias and seizures. Therefore, this acid-base balance is tightly regulated. In this article, we will look at the buffering system, urinary acid-base regulation and relevant clinical conditions.
Information on the buffering system of the blood and responses of the respiratory system can be found here.
Urinary Regulation of Acid-Base Balance
The urinary system utilises two methods to alter blood pH. Excretion of hydrogen (H+) ions as dihydrogen phosphate or ammonia and production and reabsorption of bicarbonate (HCO3–) ions.
Excretion of Hydrogen (H+) Ions
There are 2 methods by which this is achieved:
- Excretion of H+ ions in the form of dihydrogen phosphate (H2PO4–) – H+ ions are actively transported into the lumen via hydrogen-ATPase pumps on alpha-intercalated cells. Excess luminal phosphate (only 85% of total phosphate is normally reabsorbed) can bind a large portion of hydrogen ions, buffering them as H2PO4– before excretion. This excretion of H+ ions increases blood pH.
- Excretion of hydrogen ions in the form of ammonium (NH4+) – glutamine is converted to glutamate and ammonium in the proximal convoluted tubule (PCT). The ammonium dissociates to ammonia and H+ ions, allowing it to pass the through membrane and enter the lumen. Once in the lumen, it reforms ammonium by picking up a luminal H+ ion. This allows hydrogen to be excreted as ammonium ions, increasing blood pH. Furthermore, ammonia secreted at the PCT can be used further down to buffer and excrete H+ ions secreted by alpha-intercalated cells in the collecting duct. This is due to its ability to pass membranes and traverse the nephron.
Note: The glutamate created from glutamine can also go on to form bicarbonate (via its conversion to alpha-ketoglutarate) which can then be reabsorbed to further increase pH.
Bicarbonate (HCO3–) Reabsorption
Bicarbonate ions can also be reabsorbed in the PCT, which aids in the buffering system. H+ ions are secreted into the lumen via the sodium-hydrogen (Na+-H+) exchanger to combine with any filtered bicarbonate. This then forms carbonic acid (H2CO3), catalysed by carbonic anhydrase on the luminal side.
Carbonic acid then dissociates into carbon dioxide and water, which both can diffuse into the cell. Here, the reaction is undone, and carbonic anhydrase inside the cell converts carbon dioxide and water to carbonic acid, which then dissociates into H+ and HCO3– ions.
HCO3– can then be transported into the blood whilst the H+ ions can be transported back into the lumen for the cycle to repeat.
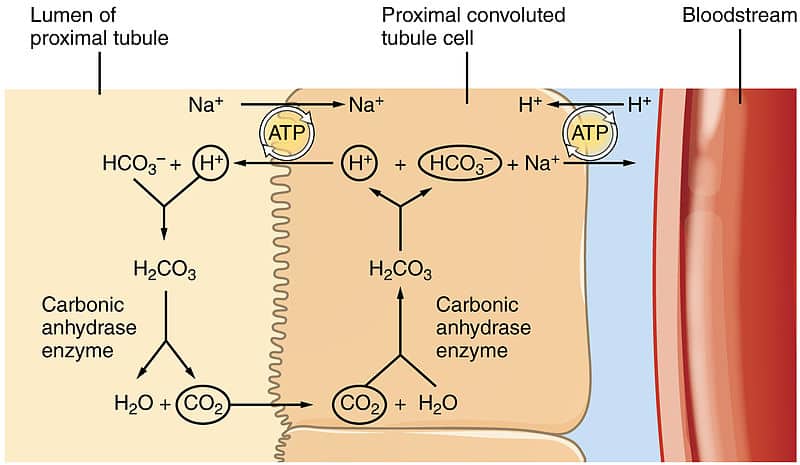
Fig 1 – Diagram showing reabsorption of bicarbonate within the kidney.
Bicarbonate (HCO3–) Production
The kidney is also able to produce bicarbonate. The metabolic activity of cells produces large amounts of carbon dioxide. This then reacts with water to produce HCO3– ions, which enter the plasma, and H+ ions are transported into the lumen.
This is useful as it also provides H+ ions to drive HCO3– reabsorption. In addition to this bicarbonate can also be produced from amino acids, which produce ammonium ions which then enter the urine.
Clinical Relevance – Anion Gap
The anion gap is a way of determining the cause of metabolic acidosis. The idea behind it is that to maintain a net neutral charge in the body, the number of positive ions (cations) and negative ions (anions) should balance out. This is calculated by using the anion gap formula:
A normal anion gap is usually 8-12mEq/L. This is because there is naturally a little bit more of the cations than the anions. However, if the amount of organic anion e.g. lactate increases then they will take up more of the anions. This means that fewer of the anions will be Cl– and HCO3– ions, so the anion gap will increase.
Clinical Relevance – Metabolic Acidosis
Metabolic acidosis is when the pH of blood < 7.35 i.e. it becomes acidic. This is caused by an increase in H+ ions, lactate, and organic acids or by a loss of HCO3–. Causes of metabolic acidosis can be divided into normal anion gap or high-anion gap.
Causes of normal anion-gap metabolic acidosis include:
- Chloride excess
- Renal tubular acidosis
- Addison’s disease
- Diarrhoea
Causes of high anion gap metabolic acidosis include:
- Lactic acidosis
- Diabetic ketoacidosis (DKA)
- Starvation
- Drugs e.g. salicylates, isoniazid, paracetamol
Symptoms of metabolic acidosis are:
- Hyperventilation – lungs try to increase pH by blowing off CO2 to reduce its concentration in blood. This is classically called Kussmaul respiration or ‘air hunger’
- Confusion
- Tachycardia
Metabolic acidosis is diagnosed through arterial blood gas analysis. Treatment involves correcting the underlying cause of acidosis, however, sometimes sodium bicarbonate may be given as well. A complication of metabolic acidosis is hyperkalaemia, which requires urgent intervention to prevent cardiac arrhythmias.
Clinical Relevance – Metabolic Alkalosis
Metabolic alkalosis is when blood pH > 7.45, making the blood alkaline. This is usually a result of a massive loss of H+ ions or increased HCO3–. Metabolic alkalosis can be caused by:
- Vomiting – causes loss of stomach acid and therefore H+ ions
- Burns
- Milk-Alkali Syndrome – leads to increased HCO3– in the blood
- Diuretic usage
The symptoms of metabolic alkalosis are:
- Hypoventilation – the lungs try to decrease pH by increasing the concentration of CO2
- Confusion
- Tetany – particularly if caused by Milk-Alkali Syndrome
- Tremor – also more common in Milk-Alkali Syndrome
Like metabolic acidosis, a diagnosis of metabolic alkalosis is made by analysing arterial blood gases.
Treatment usually involves correcting the underlying issue, but with some metabolic alkalosis, chloride replacement may also be necessary. Hypokalaemia is usually the main concern in cases of metabolic alkalosis, so this should be dealt with most urgently.