Phagocytosis is a type of endocytosis whereby a cell engulfs a particle in an internal compartment- the phagosome. The cell rearranges its membrane to surround and internalise the target particle.
Phagocytosis is a major mechanism for detecting and removing potentially pathogenic material. Phagocytes also have lysosomes which are membrane-bound organelles containing hydrolytic enzymes. These fuse with phagosomes and release their cargo into the phagosome, degrading any internalised particles.
This article will review the process of phagocytosis, highlight primary phagocytes in the immune system and discuss the clinical relevance of phagocytosis.
Phagocytic Cells of The Immune System
Many cells are capable of phagocytosis, but some immune cells are specialised for this role:
- Neutrophils are abundant in the blood and essential in acute inflammation, as they are the first immune cells to arrive at the infection site.
- Macrophages are tissue-resident cells that act as an initial defence mechanism and serve to activate the adaptive immune response.
- Dendritic cells – these cycle through the bloodstream, tissues and lymphoid organs, sampling potential pathogens and acting as a major link between the innate and adaptive immune systems.
Further information on phagocytes can be found here.
Stages of Phagocytosis
Activation
Resting phagocytes become activated by inflammatory mediators (e.g. bacterial proteins, capsules, peptidoglycan, prostaglandins, complement proteins). Consequently, they gain the ability to leave the capillaries, enter tissues and move towards the infection site (chemotaxis).
Phagocytosis requires energy as it usually involves a rearrangement of the cellular cytoskeleton (to form the pseudopodia and phagosomes) and higher levels of protein production. These processes need calcium and sodium currents and so lead to cell swelling.
Phagocytes also produce pattern recognition receptors (PRRs) which recognise and bind to pathogen-associated molecular patterns (PAMPs). PAMPs are components of pathogens and can include molecules like peptidoglycan and lipopolysaccharide (LPS).
Chemotaxis
Chemotaxis is the directional movement of the phagocyte towards a chemical attractant (chemotaxins). Chemotaxins include bacterial products (e.g. endotoxin), injured tissues, complement proteins (C3a, C4a, C5a) and chemical substances produced by leukocytes (leukotrienes). Chemotaxins both activate phagocytes and attract them to the target site to mediate their effect.
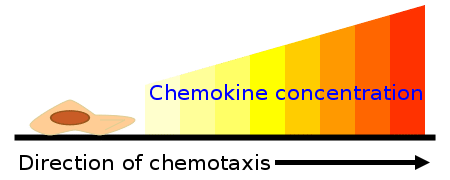
Fig 1 – Diagram showing how the chemokine concentration gradient affects the direction of chemotaxis.
Margination, Rolling and Adhesion
In margination, leukocytes assume marginal positions in the blood vessels. They intermittently stick to the walls of the venules and roll along them until they become firmly attached to the vessel wall (adhesion). At this point, they begin to move out of the vessel.
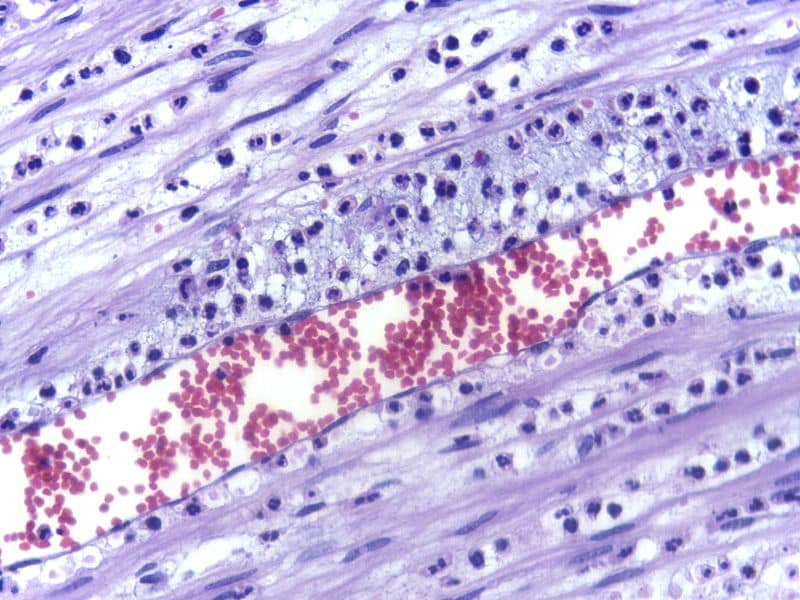
Fig 2 – Electron micrograph demonstrating margination of neutrophils in acute inflammation.
Diapedesis
This refers to the process where leukocytes ‘dig’ their way out of the venules. Leukocytes produce the enzyme collagenase, which digests the basement membrane at the attachment point, allowing them to escape.
Recognition-Attachment
There are two types enhanced and unenhanced attachment. Unenhanced attachment is when the phagocytes recognise non-specific PAMPs at the target site through their PRRs. On the other hand, enhanced attachment is more specific and efficient. In enhanced attachment, the phagocytes attach themselves to the microbe using either antibody molecules (IgG) or complement proteins (C3b, C4b).
Phagocytosis
After attachment, the phagocyte internalises the microbe into a phagosome. The phagosome then fuses with a lysosome to form a phagolysosome. Lysosomes contain digestive enzymes which can destroy the internalised material.
Pathogen killing can occur in one of two ways:
- The oxygen-dependent pathway (oxidative burst) involves the generation of reactive oxygen species (ROS) such as superoxide radicals and hydrogen peroxide. These highly reactive radical molecules react with proteins, lipids and other biological molecules to kill the pathogen.
Superoxide radicals form via the enzyme NADPH oxidase. After that, another enzyme- superoxide dismutase- converts these species to hydrogen peroxide.
Superoxide radicals can also react with the hydrogen peroxide to form powerful hydroxyl radicals, which assist in killing the invading pathogen.
- The oxygen-independent pathway involves the destruction of the pathogen via lysosomal enzymes such as proteases, phospholipases, nucleases and lysozymes. These enzymes help to kill pathogens, primarily by breaking down their cell membrane. However, this a less effective mechanism when compared to the oxygen-dependent pathway.
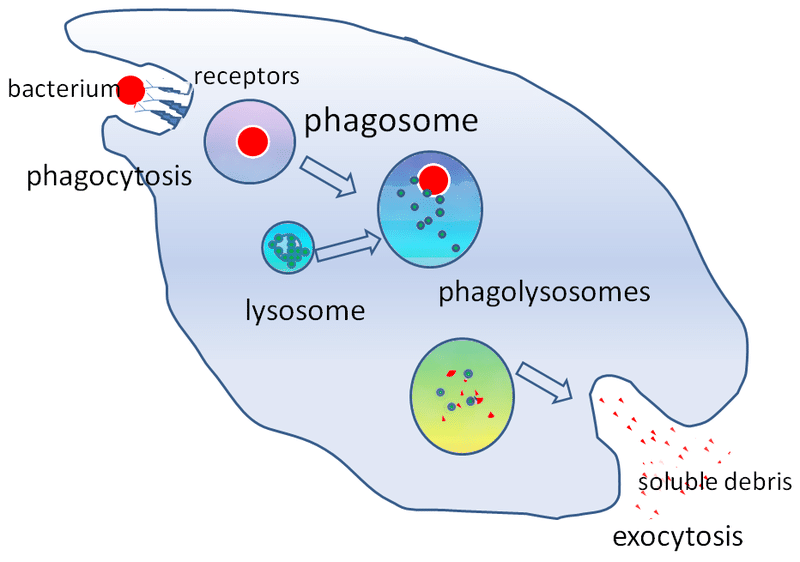
Fig 3 – Diagram summarising the process of phagocytosis.
Clinical Relevance – Chronic Granulomatous Disease (CGD)
This is a group of inherited diseases caused by an NADPH oxidase deficiency. NADPH oxidase is a key enzyme required to produce reactive oxygen species, particularly superoxide radicals.
This results in ineffective phagocytosis, as patients cannot kill pathogens via the oxygen-dependent pathway (oxidative burst). This leads to chronic inflammation and granuloma formation in many organs, as well as persistent infections.
Patients typically suffer from recurrent pneumonia episodes, skin abscesses, arthritis, cellulitis and osteomyelitis. Diagnosis usually occurs in childhood.
Management is via antibiotic therapy and immunomodulation.
