The liver is the second largest organ in the body and has a variety of important functions relating to metabolism and detoxification. Information on the anatomy of the liver can be found here.
This article will focus on the role of the liver in the metabolism of ammonia and proteins. We will also consider the clinical consequences when the liver does not function as intended, which can be described as liver failure.
Protein Metabolism
Protein synthesis occurs in all cells of the body via transcription and then translation. Protein breakdown also occurs in all cells of the body catalysed by various enzymes, which include proteases.
The liver is especially important in protein metabolism for the following reasons:
- It stores more proteins than other tissues
- Can rapidly synthesise or degrade proteins.
- It also can quickly synthesise and degrade amino acids, unlike most other tissues.
Amino Acid Synthesis
20 different amino acids are needed in the human body for protein synthesis: 10 of these can be synthesised and 10 cannot.
The 10 that cannot, are therefore essential in the diet and so are known as essential amino acids, and the other 10 are known as non-essential amino acids.
Although the synthesis of each non-essential amino acid is slightly different, they all occur by a process known as transamination, see below:
- A precursor alpha-keto acid such as pyruvate is needed
- A donor of the amino group is needed, and this is commonly glutamic acid
- An aminotransferase catalyses the reaction such as alanine transaminase (ALT) – this is one of the main liver enzymes alongside aspartate transaminase (AST)
Liver proteins
Protein synthesis is stimulated by insulin and growth hormone. Some of the most important plasma proteins are synthesised in the liver.
Plasma proteins
- Albumin
- Globulin
- Fibrinogen
- CRP (an infection marker)
- Clotting factors – Factors II, VII, IX and X are Vitamin K dependent
- Thrombopoietin
- Angiotensinogen
It is important to note that the plasma proteins synthesised by the liver play an important role in maintaining the amino acid equilibrium in the blood. In times of tissue amino acid depletion, these proteins can be degraded and released back into the blood as amino acids for tissues to use in protein synthesis.
It is also vital to note that the plasma proteins provide oncotic pressure in the blood, meaning they hold water in the plasma.
Clotting factors
Almost all the clotting factors in the blood are synthesised by the liver.
For this reason, liver disease can lead to a tendency for bleeding, as fewer clotting factors are produced. For more detail, see our article on the coagulation cascade here.
In addition to this, vitamin K is essential to the carboxylase enzyme that is used to make clotting factors II (prothrombin), VII, IX, X and protein C.
As vitamin K is fat-soluble, bile is needed for its digestion and absorption. In biliary obstruction, Vitamin K is not appropriately absorbed and thus the Vitamin K dependant clotting factors are not adequately synthesized, leading to a further tendency towards bleeding.
Amino acid catabolism
Once the cells of the body have reached their limit of protein storage, the left-over amino acids in the blood are metabolised in the liver, prior to their conversion into lipids or glucose. This is done through the processes of transamination and deamination, before the ammonia generated is cleared in the urea cycle. We will now consider this in more detail:
Transamination
Through transamination, amino acids are converted into keto acids, using an acceptor to accept the amine group. This is catalysed by aminotransferases such as ALT/AST. For example:
- Amino acid + a-ketoglutarate –> glutamate and keto acid
- Amino acids + oxaloacetate –> aspartate and ketoacid
Aspartate can then be metabolised further:
- Aspartate + a-ketoglutarate –> oxaloacetate and glutamate
The end result of this step is the generation of glutamate.
Deamination
Glutamate is metabolised further by glutamate dehydrogenase. The amine group is removed and rapidly forms ammonia (NH3) and subsequently ammonium (NH4+), alongside a-ketoglutarate. The ammonium is highly toxic and must therefore be removed through the urea cycle. Meanwhile, the a-ketoglutarate can enter the TCA cycle.
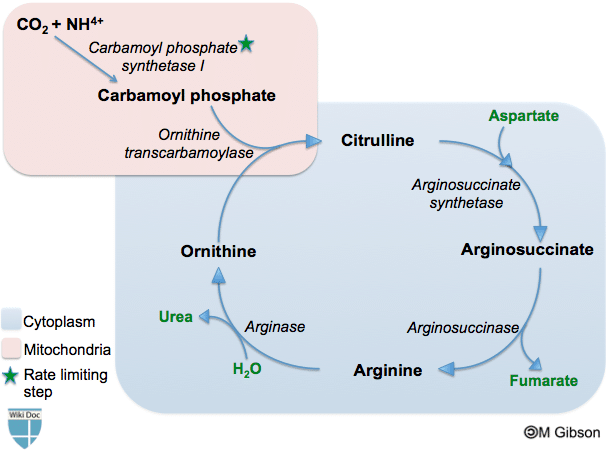
Urea cycle
The urea cycle occurs both within the mitochondria and cytoplasm of the hepatocyte. Within the mitochondrion, ammonia reacts with ATP and CO2 to generate citrulline from ornithine. The citrulline then enters the cytoplasm and passes through a series of reactions, consuming aspartate along the way, to regenerate ornithine. The ornithine then re-enters the mitochondrion and the cycle repeats.
Through this process, the toxic ammonia is converted into harmless urea, which is easily excreted.
Vitamin Metabolism
The liver is important in the metabolic activation of Vitamin D. It is carried to the liver in the blood where it is first converted to the prohormone calcifediol via hydroxylation. This calcifediol is then transported to the kidneys where it is converted into calcitriol, the biologically active form of Vitamin D.
The conversion of calcifediol to calcitriol is catalysed by 25-hydroxyvitamin D3 1-alpha-hydroxylase. This conversion is stimulated by parathyroid hormone and low calcium.
Clinical Relevance – Liver Failure
Pathophysiology
Liver failure may occur due to a variety of reasons such as hepatitis B, hepatitis C, alcoholism, paracetamol overdose etc. It may happen in a previously healthy liver- acute liver failure, but more commonly happens on the background of cirrhosis- chronic liver failure.
In liver failure essentially all functions are compromised, and the signs and symptoms are related to a deficiency in these functions.
Signs and Symptoms
Bleeding – the liver synthesises clotting factors for coagulation. Additionally, vitamin K-dependent clotting factors are particularly affected due to the decreased absorption of vitamin K that occurs in liver disease. As a result, patients might bruise easily and have a raised PT and INR.
Oedema & ascites – the liver synthesises almost all the body’s plasma proteins, without which the oncotic pressure of the blood falls, and fluid leaks out of the blood vessels into the extracellular space, leading to oedema. Additionally, cirrhosis increases resistance to the flow of blood which causes portal hypertension. The hypertension in the portal vein leads to yet more fluid leaking out of the portal vein contributing to ascites.
GI bleeds – portal hypertension can also lead to varices (enlargement of the oesophageal veins) which can rupture and bleed. This is also coupled with an overall tendency to bleed due to diminished clotting factors.
Fetor hepaticus (faecal/sweet-smelling breath) – this is a late sign and it thought to be due to thiols (sulphur-containing compounds) and to a lesser extent acetone on the breath. Thiols accumulate in the blood as portal vein hypertension leads to portosystemic shunting, meaning some thiols absorbed by the gut escape first pass metabolism. Raised acetone levels are due to an increase in fatty acid breakdown, as a response to impaired gluconeogenesis and glycogen storage.
Jaundice – damaged hepatocytes have a decreased ability to conjugate bilirubin and secrete it into to the bile canaliculi, thus leading to high levels of bilirubin in the blood which in turn leads to deposition in the skin and sclera giving them a yellow appearance.
Hepatic encephalopathy– damaged hepatocytes do not metabolise nitrogenous waste efficiently and some nitrogenous waste absorbed by the gut is shunted to the IVC due to portal vein hypertension. As a result, there are increased ammonia levels in the blood, and ammonia crosses the blood-brain barrier where it is metabolised by astrocytes to form glutamine. Glutamine increases the osmotic pressure in the brain, leading to brain oedema. A classic sign of hepatic encephalopathy, aside from the obvious confusion, agitation and vomiting, is asterixis (the liver flap).