The complement system, also known as the complement cascade, forms a part of the innate immune system. Complement components are generally made in the liver and circulate in their inactive form until they are needed.
The overall aim of the complement system is to support other parts of the immune response by opsonising pathogens and triggering inflammation.
This article shall cover the activation of the complement system, its roles in the immune response and relevant clinical conditions.
Activation of the Complement System
There are three ways to activate the complement system, involving different molecules initially but converging to produce the same effector molecules. Each involves activation of enzymes that cleave their substrates to form a cascade, so that the complement response is amplified.
- The Classical Pathway
- The Mannose-Binding Lectin Pathway
- The Alternative Pathway
All three pathways produce C3 convertase, an enzyme which triggers further effects downstream. The effects of C3 convertase are discussed below.
The Classical Pathway
The classical pathway is activated when a complement protein called C1q binds either directly to a pathogen, or onto an antigen-antibody complex. This will then trigger cleavage of the subsequent complement proteins in the cascade, resulting in production of C3 convertase and it’s downstream effects.
Its involvement in antigen-antibody complexes means it has a role in the adaptive immune response as well as the innate.
The Mannose-Binding Lectin (MBL) Pathway
Mannose-Binding Lectin (MBL) is a protein produced in the liver. Its role is to detect carbohydrates containing mannose on the surface of pathogens, activating a protease called MASP. MASP is responsible for cleaving complement components, which activates a similar cascade to the classical pathway, eventually producing C3 convertase.
The Alternative Pathway
The alternative pathway is usually activated by bacterial endotoxin, a lipopolysaccharide present on the outer membrane of gram negative bacteria. This results in spontaneous hydrolysis of C3 into small amounts of factor C3b, which combines with other factors to produce C3 convertase.
Immune Effects of the Complement System
Whichever way C3 is activated it will then activate C5, which in turn activates C6, C7, C8 and C9 in a cascade. As such even a small signal can lead to the rapid activation of many thousands of complement molecules – this is important in the immune response as pathogens are also able to replicate very quickly within the body.
Once activated the complement system has several effects, including:
- Opsonisation
- Lysis of pathogens
- Chemotaxis
- Inflammation
Opsonisation
C3 convertase is a product of all the pathways triggering the complement cascade and is responsible for converting factor C3 into C3a and C3b. C3b binds to antigens on the pathogen, which stimulates neutrophils and macrophages to phagocytose pathogens – this is called opsonisation.
Lysis of Pathogens
Lysis of pathogens is facilitated by the formation of the membrane attack complex (MAC). C3 convertase is vital to the production of the MAC because it generates C3a and C3b. C3b combines with other factors to produce C5 convertase, an enzyme which converts factor C5 to C5a and C5b.
C5b combines with several factors to produce the MAC. The MAC ruptures the bacterial cell membrane, allowing fluid to enter the bacteria and causing cell lysis. However, because they possess a cell wall, gram positive bacteria and fungi do not swell and hence cannot be lysed by the complement system.
Chemotaxis
The production of C5a by C5 convertase attracts neutrophils and macrophages to the site of infection and causes extravasation of leucocytes from capillaries to tissues. C3a is another complement component that acts as a chemotaxin.
Inflammation
C3a, C4a and C5a are the complement components responsible for causing inflammation. They bind to mast cells and basophils to cause degranulation. The histamine and serotonin released increase vascular permeability. C3a, C4a and C5a also promote synthesis of pro-inflammatory cytokines.
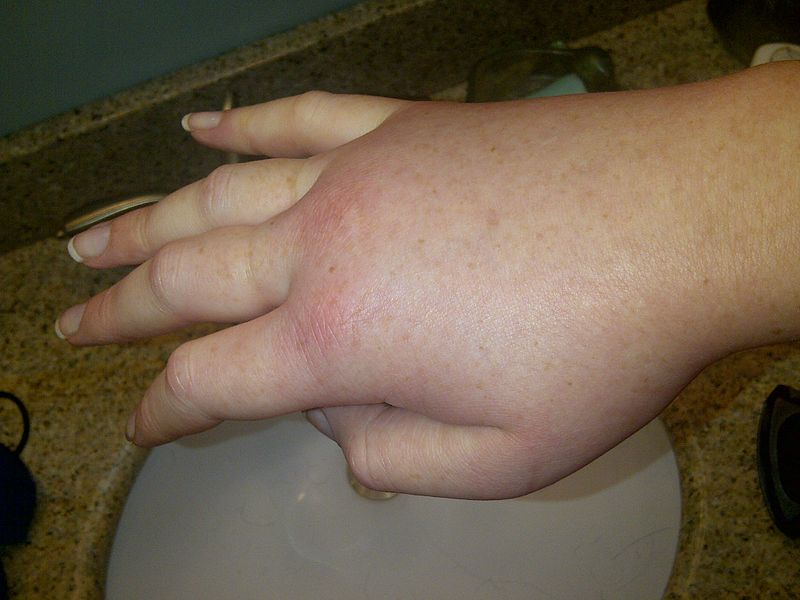
Clinical Relevance – Hereditary Angioedema
Hereditary angio-oedema is a condition caused by a deficiency of C1 esterase inhibitor. C1 esterase inhibitor is responsible for inhibiting the classical and MBL pathways.
A deficiency of the enzyme leads to excess production of inflammatory mediators such as bradykinin, causing leakage of plasma into the extracellular space. It can present with localised subcutaneous swelling as well as facial, lip or mouth swelling.
It is considered a medical emergency as this can potentially progress to laryngeal oedema, which has a high mortality. In severe cases, the intestines can also be affected, resulting in abdominal cramps, vomiting or abdominal distention.