The mononuclear phagocyte system (MPS) is an important part of the innate immune system. It serves as a network of phagocytic cells in the blood and lymphatic system as well as the lymph nodes, liver and spleen. It was previously known as the reticuloendothelial system.
In this article, we will discuss the composition and function of the mononuclear phagocyte system, and consider its clinical relevance.
Functions of the MPS
The main role of the MPS is to identify foreign antigens and mount an appropriate immune response. These antigens are phagocytosed before they can cause further harm to the body.
Additionally, the MPS plays a key role in the destruction of old and dysfunctional cells, which allows the body to recycle key materials such as iron.
Cells of the MPS
The primary cell of the MPS is the phagocyte. Phagocytes surround foreign antigens and damaged cells, before destroying them by a process known as phagocytosis.
The most common phagocytes are macrophages, dendritic cells (derived from monocytes) and granulocytes.
Monocytes are formed in the bone marrow and circulated in the blood, from where they migrate into surrounding tissues. Here, they further mature to become either tissue histiocytes or macrophages.
Macrophages are further categorised according to the organ they reside in:
- CNS – Microglial cells.
- Liver – Kupffer cells.
- Lungs – Alveolar macrophages.
- Skin and mucosa – Langerhans cells.
Organs of the MPS
The Spleen
The spleen is formed from both red pulp and white pulp. The red pulp contains endothelial macrophages, which play a key role in ensuring that defective or ageing red blood cells are phagocytosed. This allows any dysfunctional red blood cells to be disposed of, and the iron within them can be recycled.
The spleen also serves as a pool for platelets and red blood cells, which can be rapidly mobilised when needed.
The white pulp is largely responsible for the immunological function of the spleen and, as it is one of the organs of the lymphatic system that contains B and T lymphocytes. Any antigen-presenting cell passing through the spleen can stimulate activation of lymphocytes to mount an appropriate immunological response.
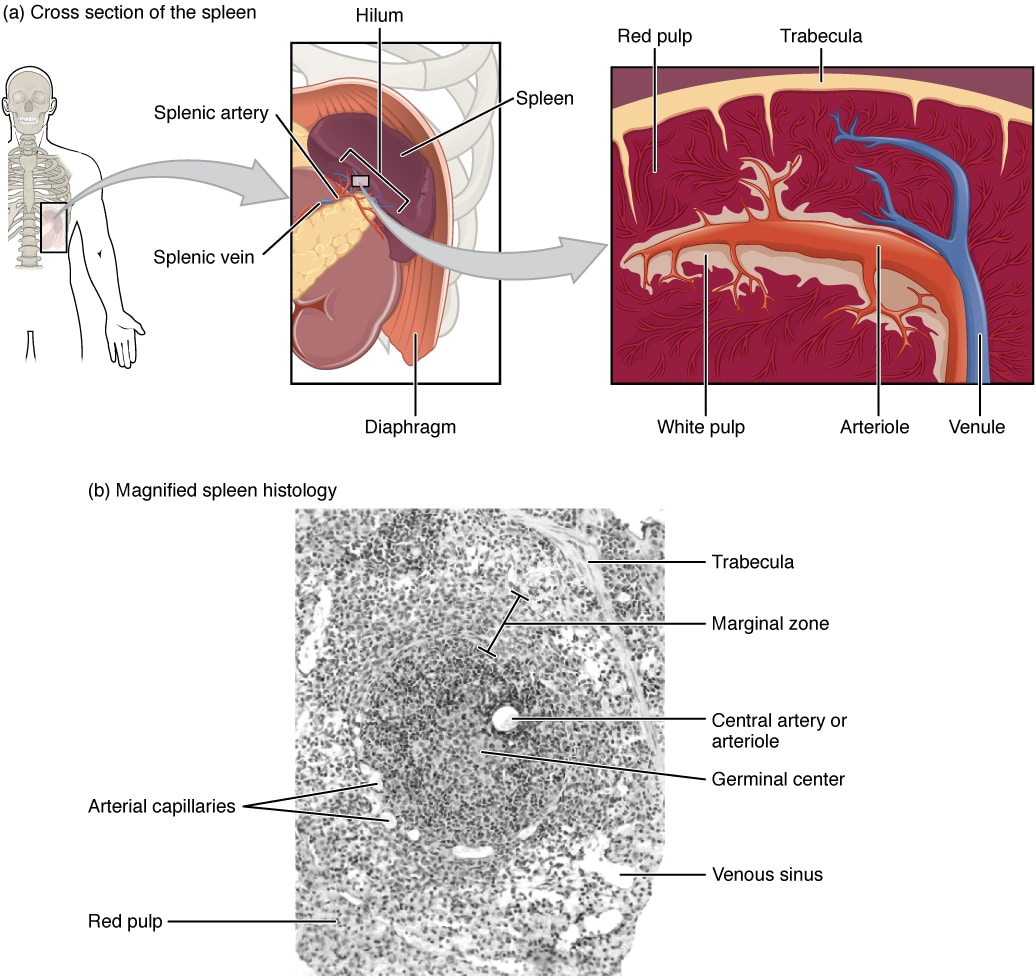
Fig 2 – Diagram showing the anatomical location of the spleen, a cross-sectional diagram and magnified histology.
The Lymphatic System
The lymphatic system consists of lymphatic vessels and lymph nodes which act to filter tissue fluid from the blood. Lymph nodes house B and T lymphocytes which detect pathogens, leading to a specific immune response.
More information about the lymphatic system can be found on our sister site, here.
The Liver
As previously discussed, the liver contains Kupffer cells which reside in the vessels forming the capillary beds of the liver, known as sinusoids.
As blood enters the liver via the portal vein and drains into the sinusoids, the Kupffer cells remove foreign materials through phagocytosis. Additionally, they stimulate a local inflammatory response using cytokines and oxygen radicals.
Kupffer cells are also involved in the metabolism of red blood cells and haemoglobin. The haem portion is further broken down into iron for immediate reuse or for storage, whilst the globin chains are reused. Bilirubin is conjugated here and secreted in the bile.
For more about the liver, see here.
Clinical Relevance – Hyposplenism
Damage to the spleen predisposes to infection by encapsulated bacteria. This damage can occur directly following trauma, surgery or sickle cell anaemia. Alternatively, a state of functional hyposplenism can occur in diseases such as coeliac disease.
Splenic macrophages are responsible for the clearance of blood-borne bacteria. Encapsulated bacteria can only be phagocytosed following opsonisation, and are poorly cleared otherwise. C3b is an example of an opsonin which facilitates this clearance, and itself is activated by IgM.
If the spleen is damaged or dysfunctional, splenic macrophages are not present to remove these opsonised encapsulated bacteria from the circulation. As a result, the bacteria persist in the bloodstream, hence pre-disposing to sepsis.