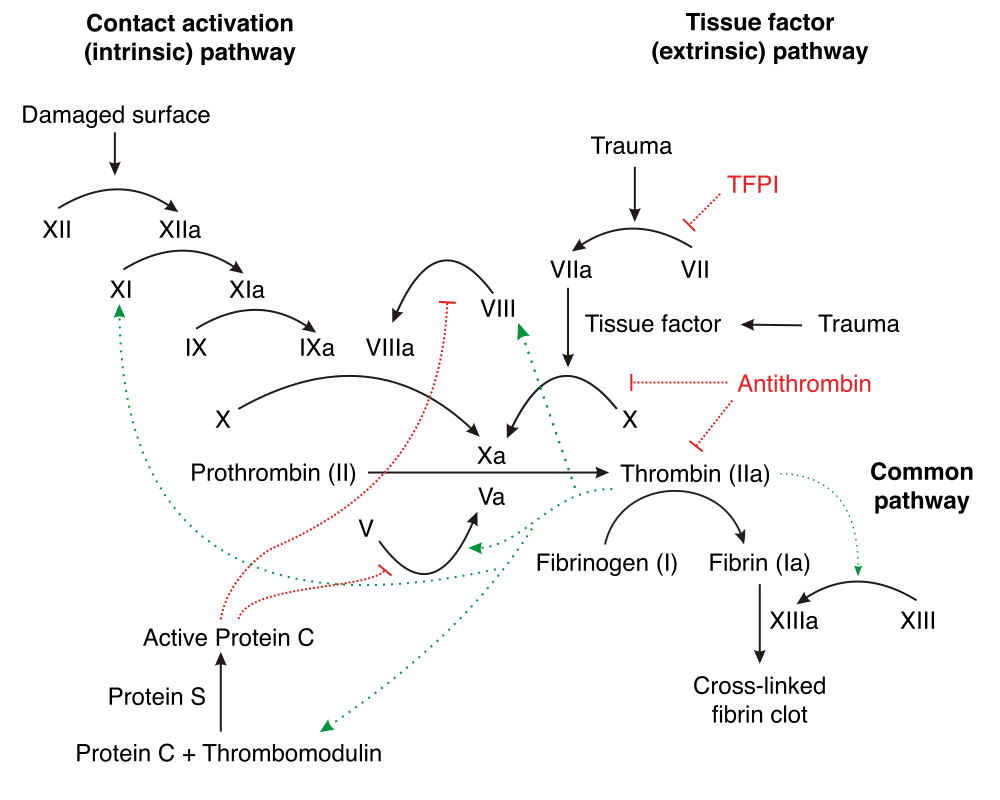
Coagulation is the formation of a blood clot, and is essential to haemostasis. Haemostasis is the body’s physiological response to damaged blood vessels, to slow down, minimise and eventually cease the bleeding.
The coagulation process is characterised by a cascade of events which lead to the formation of a blood clot. Proteins called clotting factors initiate reactions which activate more clotting factors.
This process occurs via two pathways which unite downstream to form the common pathway. These are:
- The extrinsic pathway: This is triggered by external trauma which causes blood to escape the circulation
- The intrinsic pathway: This is triggered by internal damage to the vessel wall.
Extrinsic Pathway of Coagulation
The extrinsic pathway unfolds as follows:
- Damage to the blood vessel means that factor VII exits the circulation into surrounding tissues
- Tissue factor (factor III) is released by damaged cells outside the circulation
- Factor VII and factor III form a complex, known as the TF-VIIa complex.
- TF-VIIa then activates factor X into its active form, factor Xa
- In conjunction with factor Va, this triggers the formation of thrombin.
Note that the extrinsic pathway is believed to be responsible for the initial generation of activated Factor X (Factor Xa), whereas the intrinsic pathway amplifies its production.
Intrinsic Pathway of Coagulation
The intrinsic pathway is the longer and more intricate pathway:
- Factor XII is activated once it comes into contact with negatively charged collagen on the damaged endothelium, triggering the cascade as detailed in Figure 1.
- Along with clotting factors, platelets form a cellular ‘plug’ at the site of injury. These platelets also release mediators that facilitate further clotting, including Factor VIII.
- Factor IX combines with Factor VIII to form an enzyme complex that activates factor X, which along with factor Va, stimulates the production of thrombin.
Common Pathway of Coagulation
The intrinsic and extrinsic pathways converge to give rise to the common pathway. The activated factor X causes a set of reactions resulting in the inactive enzyme prothrombin (also called factor II) being converted to its active form thrombin (factor IIa) by Prothrombinase.
The thrombin then converts soluble fibrinogen (also referred to as factor I) into insoluble fibrin strands. The fibrin strands which comprise the clot are stabilised by factor XIII.
Regulation of Clotting
To prevent excessive clotting and subsequent disease, mediators including Protein C and Protein S provide negative feedback on the clotting cascade.
Protein C is activated following contact by thrombomodulin, which is itself activated by thrombin. Along with co-factors including protein S, activated protein C degrades factor Va and factor VIIIa, thus slowing the rate of clotting.
Calcium ions play a role through their interaction with the activation of several clotting factors. Low levels of calcium are therefore inhibitory to the clotting cascade.
Antithrombin is a protease inhibitor that degrades thrombin, factor IXa, factor Xa, factor XIa and factor XIIa. It is constantly active but can be activated further by a group of common anticoagulants known as heparins.
Clinical Relevance – Protein C Deficiency
Protein C deficiency can either be inherited through a range of mutations or can be acquired in disease states including sepsis and liver disease.
Due to the reduction in protein C, the clotting cascade is under less inhibition. Consequently, patients are pre-disposed to abnormal and excessive clotting, leading to illnesses including deep vein thrombosis and stroke.
Restoring Blood Flow – Fibrinolysis
In order for blood flow to be re-established as the blood vessel heals, the thrombus must eventually be degraded. During fibrinolysis, fibrin is dissolved leading to the consequent dissolution of the clot.
The endothelial cells of the blood vessel wall secrete tissue plasminogen activators (tPAs) which convert the precursor plasminogen into plasmin. Plasmin subsequently cleaves the fibrin within the thrombus, leading to its degradation.
tPAs are released very slowly following trauma from the endothelial cells, and therefore there is a substantial time delay until there is a sufficient concentration for fibrinolysis.
Clinical Relevance
Haemophilia
It is an inherited bleeding disorder caused by a partial or total deficiency in specific clotting factors. There are 3 types of Haemophilia:
- Type A: deficiency in factor VIII
- Type B: deficiency in factor IX
- Type C: deficiency in factor XI
Haemophilia A and B are both X-linked disorders and are therefore most prevalent in males. Since this condition impairs the body’s normal clotting response, people with haemophilia may bruise easily, bleed spontaneously or bleed for longer following surgery and injury.
The manifestation of bleeding is related to the severity of the clotting factor deficiency. Therapy for these patients includes the replacement of the missing clotting factor.
Thrombolysis
Abnormal or unwanted blood clots in the circulation can lead to infarction. In the brain this is known as an ischaemic stroke; in the heart, this is known as a myocardial infarction.
In order to degrade the blood clot, one treatment option is thrombolysis. This involves using synthetic tPAs to artificially destroy the clot. One obvious, but common, side effect is unwanted bleeding at other sites.