Cellular receptors are proteins which are essential for cell signalling. When a specific signalling molecule (ligand) binds to its corresponding receptor, this acts like a key unlocking a door. The binding of a ligand triggers a change in the receptor, which leads to a host of downstream signalling actions and changes inside the cell.
While there are many different types of receptors, they can be broadly classified into cell surface receptors and intracellular receptors. This article will discuss the structure and function of the main types of receptors, with examples of their clinical relevance.
Cell Surface Receptors
Cell surface (or transmembrane) receptors span the cell membrane and provide cellular access for ligands that cannot cross the plasma membrane themselves. This is often because these ligands are hydrophilic or large, making them unable to diffuse through the plasma membrane.
There are three main types of cell surface receptors: ion channel receptors, G-protein coupled receptors (GPCRs) and enzyme-linked receptors. While these each have unique structures, cell surface receptors are generally composed of three domains: an extracellular ligand-binding domain, a hydrophobic domain embedded within the plasma membrane, and an intracellular domain.
Ion Channel Receptors
Ion channel receptors are like gates which open to provide ions with entry into the cell. Different subtypes exist, for example, voltage-gated ion channels, which open or close in response to changes in membrane potential.
Another subtype, ligand-gated ion channels, open or close in response to the binding of a specific ligand (for example a neurotransmitter) to their extracellular domain. When they open, they provide a channel through which ions can passively enter the cell.
Ion channels are crucial for many processes, for example in the nervous system. Transmission of action potentials from one neuron to the next depends on the flow of sodium and potassium ions via voltage-gated ion channels.
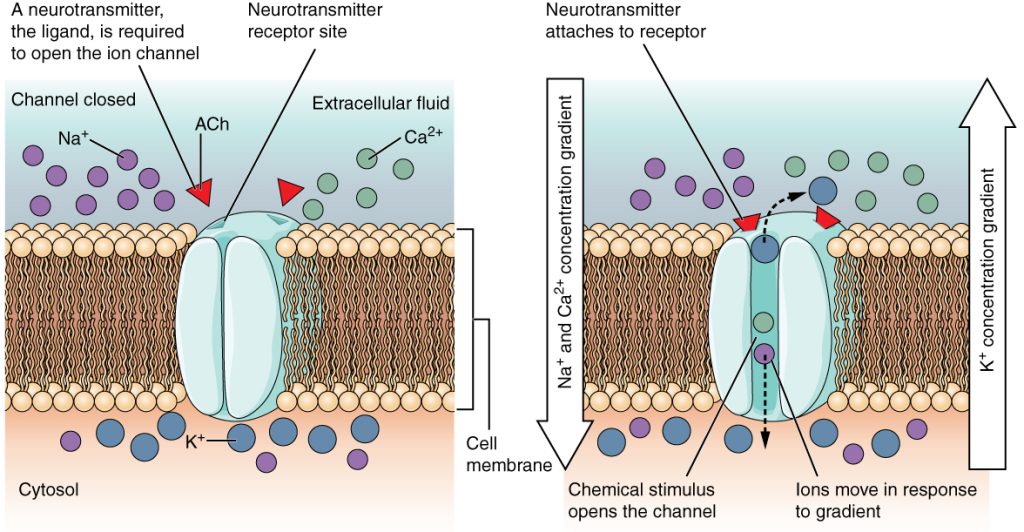
Fig 1 – Diagram showing the function of ligand gated ion channels
G-protein Coupled Receptors
GPCRs are a diverse group of cell surface receptors that use specific proteins called G proteins to participate in cell signalling. Their structure and function are discussed in detail in this article.
Enzyme-linked Receptors
Enzyme-linked receptors are cell surface receptors that have a catalytic site on their intracellular domain, which is either associated with an enzyme or has enzymatic activity itself, enabling it to catalyse chemical reactions.
The binding of a ligand to the extracellular domain leads to activation of the enzyme and triggers a specific response. An important subtype of enzyme-linked receptors is tyrosine kinase receptors.
Tyrosine kinase receptors contain kinase – an enzyme which transfers phosphate molecules. In the case of tyrosine kinase, phosphate molecules are transferred to the amino acid tyrosine. When a ligand binds to the extracellular domain of a tyrosine kinase receptor, the receptor dimerises with its neighbouring receptor.
Dimerisation triggers phosphorylation of the tyrosine molecules on the intracellular domain of the receptor. Phosphorylated tyrosine molecules are involved in many different signalling cascades which are crucial for various processes such as cell division and wound healing.
Intracellular Receptors
Intracellular (or internal) receptors are found inside the cell, in the cytoplasm or nucleus. Their ligands tend to be small, hydrophobic (i.e. lipid-soluble) molecules, which can diffuse across the plasma membrane by themselves.
Once inside the cell, these ligands bind to their specific intracellular receptor to form a ligand-receptor complex, which will then bind to DNA in the nucleus and directly affect gene transcription and protein synthesis.
Like cell surface receptors, intracellular receptors also have three core domains. These comprise a ligand-binding domain, DNA binding domain and an amino terminus (which interacts with the gene transcription machinery).
Examples of ligands which bind to intracellular receptors include sex hormones, thyroid hormones and fat-soluble vitamins (A, D, E and K).
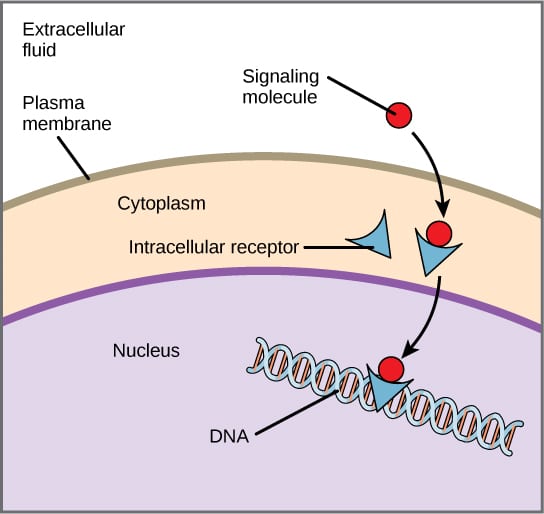
Fig 2 – Diagram of intracellular receptor action
Clinical Relevance – Receptor Dysfunction and Cancer
Tyrosine kinase receptors are essential for much of the growth signalling and cellular differentiation in the body. Many growth factors work in tandem with tyrosine kinase receptors to bring about cell division. While critical for cell survival and proliferation, tyrosine kinase signalling must be tightly regulated because dysregulation is associated with certain cancers.
Chronic myeloid leukaemia (CML) is caused by a mutation which results in increased tyrosine kinase activity. This leads to increased proliferation of myeloid cells in the bone marrow. Treatment for CML has been revolutionised by tyrosine kinase inhibitors such as imatinib, which specifically block cancer cell growth while sparing the non-mutated cells.
Clinical Relevance – Viral Entry Into Cells
Endogenous ligands bind to receptors to gain access into cells, but viruses can also do the same. Instead of receptor binding triggering a physiological signalling cascade, a virus binding to, and hijacking, a receptor facilitates infection by granting the virus entry into the cell.
For example, HIV infects CD4 T cells and macrophages by binding to their CD4 receptor. SARS-CoV-2, the cause of COVID-19, enters cells by binding to angiotensin-converting enzyme 2, a receptor found in various organs including the lung, heart and kidneys.
