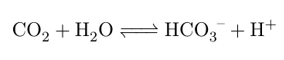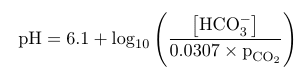The acid-base balance is vital for normal bodily functions. Disruption of this equilibrium can lead to severe complications such as arrhythmias and seizures. Therefore, this balance is tightly regulated. In this article, we will look at the buffering system, responses of the respiratory system and relevant clinical conditions.
Buffering System
Blood has the ability to be resistant to small changes in pH, a characteristic known as “buffering”. This is due to the basal levels of bicarbonate and hydrogen ions in blood. The chemical reaction is given by:
This reaction can be used to control pH. For example, in metabolically active tissues, there is an increase in hydrogen ions. These can react with bicarbonate in the red blood cells to form carbon dioxide which can then be exhaled by the lungs. The compensatory systems of the body rely on this equation. This will be discussed in more detail later.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation relates the pH to the ratio between the concentration of bicarbonate and the partial pressure of carbon dioxide. It is given by:
This shows that the ratio between bicarbonate production and partial pressure of carbon dioxide drive the pH levels of the blood. By increasing bicarbonate levels, the pH will rise and turn more alkaline. By increasing the partial pressure of carbon dioxide, the pH of blood will fall and turn acidic. The usual range of blood pH is from 7.35 to 7.45. When pH levels drop below 7.35, it is said to be acidotic, and when pH levels rise above 7.45 it is said to be alkalotic.
How is Balance Restored?
When blood pH deviates from the normal range, there are two body systems which are activated to restore equilibrium. The respiratory system alters the respiratory rate, to change the concentration of carbon dioxide in the blood, whilst the urinary system changes the reabsorption or production of bicarbonate or hydrogen ions. This is known as “compensation”.
Information on the response of the urinary system can be found here.
Respiratory Responses
There is a complex regulatory mechanism for changing the respiratory rate. Chemoreceptors detect the levels of certain molecules in the blood, and alter the respiratory rate accordingly. Peripheral chemoreceptors, in the carotid sinus and aortic arch, signal to the brain stem via cranial nerves to alter the respiratory rate. Central chemoreceptors function via a different method. When there is a rise in carbon dioxide in the blood, it can diffuse into the cerebrospinal fluid (CSF) as it is a small molecule. An enzyme called Carbonic Anhydrase can then turn carbon dioxide and water into bicarbonate and hydrogen ions. Hydrogen ions are then sensed by chemical chemoreceptors which alter the respiratory rate directly.
Further information on the role of chemoreceptors can be found here.
Clinical Relevance
Respiratory Acidosis
Respiratory acidosis is where there is an increase of carbon dioxide in the blood, the cause of which is due to a disorder in the respiratory system. Common causes include respiratory depression by opiates, disorders of the respiratory muscles such as in polio and airway obstructions such as in sleep apnoea. This overwhelms the buffering systems and causes a drop in pH. Therefore, the kidneys have to excrete more hydrogen ions (via the methods discussed previously) in addition to increasing bicarbonate reabsorption.
Respiratory Alkalosis
Respiratory alkalosis is associated with hyperventilation, which can occur due to hypoxaemia (eg. from high altitudes) or a pulmonary embolism. The compensatory methods for respiratory alkalosis is the opposite of respiratory acidosis. Due to the high levels of bicarbonate, hydrogen ions are reabsorbed to attempt to bring the pH down by decreasing hydrogen excretion and decreasing bicarbonate reabsorption and production.