In order for the immune system to protect the body from pathogens, immune cells interact to identify the presence of foreign antigens. These foreign antigens act as red flags.
Normally, immune cells only react against foreign antigens, and healthy cells displaying self-antigens do not trigger an immune response. Immune tolerance refers to the unresponsiveness of the immune system to self-antigens. This is crucial in order to avoid inflammatory reactions against healthy tissue. Autoimmunity arises when there is a breakdown of immune tolerance.
In this article, we will consider the mechanisms which maintain immune tolerance, and we will discuss the consequences of these mechanisms failing.
Mechanisms of Immune Tolerance
The innate immune system provides non-specific protection against anything it identifies as foreign by recognising common pathogenic features. Conversely, the adaptive immune system is specific, with each B and T lymphocyte receptor recognising a unique foreign antigen. The vast diversity of B and T lymphocyte receptors enables the immune system to recognise a wide range of pathogens. However, it inevitably results in the generation of self-reactive lymphocytes.
Central Tolerance
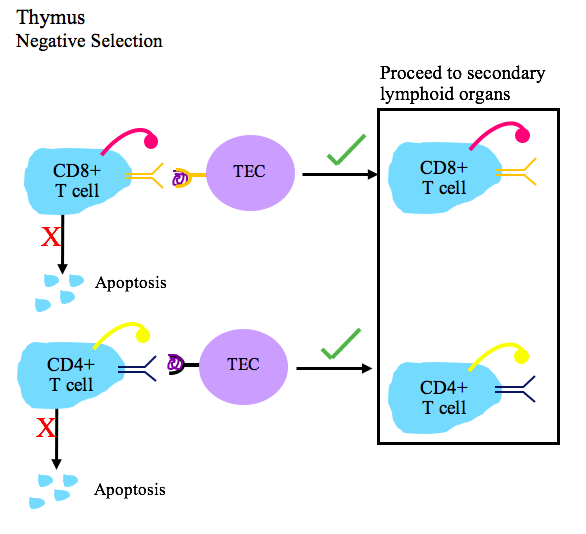
To prevent self-reactive lymphocytes from causing damage, there are various checkpoints that maintain immune tolerance. Central tolerance is the first checkpoint. This is the process by which self-reactive B and T lymphocytes are destroyed in the primary lymphoid organs, namely the bone marrow and the thymus).
For example, T lymphocytes developing in the thymus are exposed to self-antigens from a range of tissues, such as proteins from the liver, pancreas and thyroid gland. This ‘educates’ them about self-antigens. T lymphocytes with receptors that react strongly with self-antigens are destroyed through a process called negative selection. Only those that do not react strongly with self-antigens are able to proliferate and leave the thymus to circulate throughout the body.
Peripheral Tolerance
While central tolerance destroys most self-reactive lymphocytes, it is not a flawless process. Some self-reactive lymphocytes escape negative selection and enter the circulation, where they can cause tissue injury unless they are destroyed or suppressed in the peripheral tissues. Therefore, peripheral tolerance is the next checkpoint in an attempt to prevent autoimmunity.
It includes deletion of self-reactive lymphocytes by apoptosis, suppression of self-reactive lymphocytes by regulatory T cells (Tregs – a specific type of T lymphocyte with immunosuppressive effects), and induction of anergy (rendering lymphocytes unresponsive to self-antigens).
Interestingly, Tregs also play a crucial role in protecting the foetus, which has foreign paternal antigens, from immune attack by maintaining maternal immune tolerance during pregnancy.
Loss of Immune Tolerance
Despite all of these checkpoints, some people still develop autoimmune diseases. These are thought to arise due to a combination of genetic and environmental factors. Some of the main risk factors include:
- Genetic predisposition – autoimmunity often run in families, with the human leukocyte antigen (HLA) gene family contributing to half of genetic predispositions.
- Female sex – many autoimmune diseases are much more common in females of childbearing age, suggesting that oestrogen may affect the immune response.
- Impaired Treg response – in certain autoimmune diseases, Treg numbers may be low or have impaired function.
- Infection – autoimmune conditions may be triggered by infection. One interesting mechanism for this is molecular mimicry, in which the immune system fails to distinguish between certain foreign antigens that are structurally similar to self-antigens and attacks the self-antigens as well.
Clinical Relevance – Molecular Mimicry
Clinical examples of molecular mimicry include:
- Rheumatic fever – This develops after group A streptococcal infection such as strep throat or scarlet fever. This is due to antibodies made against the bacteria cross-reacting with structurally similar self-proteins in the heart.
- Guillan-Barré syndrome – Often follows gastroenteritis with Campylobacter jejuni, with antibodies cross-reacting with components of nerve cell membranes.
Features of Autoimmunity
Autoimmune diseases/autoimmunity affect around 5% of the population and include a diverse range of more than 80 conditions, from rheumatoid arthritis to pernicious anaemia. While there are notable exceptions, autoimmune diseases tend to have certain features in common. These include:
- HLA genetic association
- Higher incidence among females
- Onset in young adulthood or middle age
- Detectable autoantibody levels
- Positive response to immunosuppressive treatments
- Fluctuations in symptom severity, with flare-ups and remissions
The spectrum of autoimmune diseases ranges from organ specific to non-organ specific conditions, based on whether the self-antigen that the immune system is attacking is only present in specific tissues or widely distributed throughout the body.
For example, Hashimoto’s thyroiditis is a classic organ specific condition, with the autoimmune response limited to the thyroid gland. In this condition, there are anti-TPO antibodies. On the other end of the spectrum is systemic lupus erythematosus (SLE).
Systemic lupus erythematosus (SLE) is a systemic condition caused by autoantibodies. These target structures found in nearly all cell types, such as double stranded DNA. For this reason, SLE can cause an array of seemingly unrelated and non-specific symptoms, which can make it difficult to diagnose.
Clinical Relevance – Sjögren’s Syndrome
Sjögren’s syndrome is an autoimmune disease that typically presents in middle-aged women. Autoantibodies mainly target the lacrimal and salivary glands, resulting in dry eyes and mouth. The antibodies implicated are anti-Ro and anti-La. Other symptoms may include fatigue, joint pain and rashes. Management focuses on symptom relief, such as artificial tears and good oral hygiene, and immunosuppression for severe cases.
Clinical Relevance – Pernicious Anaemia
Pernicious anaemia is an autoimmune disease caused by autoantibodies against intrinsic factor or gastric parietal cells. Intrinsic factor is a protein required for vitamin B12 absorption. Therefore, a lack of intrinsic factor results in vitamin B12 deficiency. Signs and symptoms include anaemia (fatigue, weakness), peripheral neuropathy (tingling, numbness) and glossitis (swollen tongue). Treatment involves regular vitamin B12 injections.