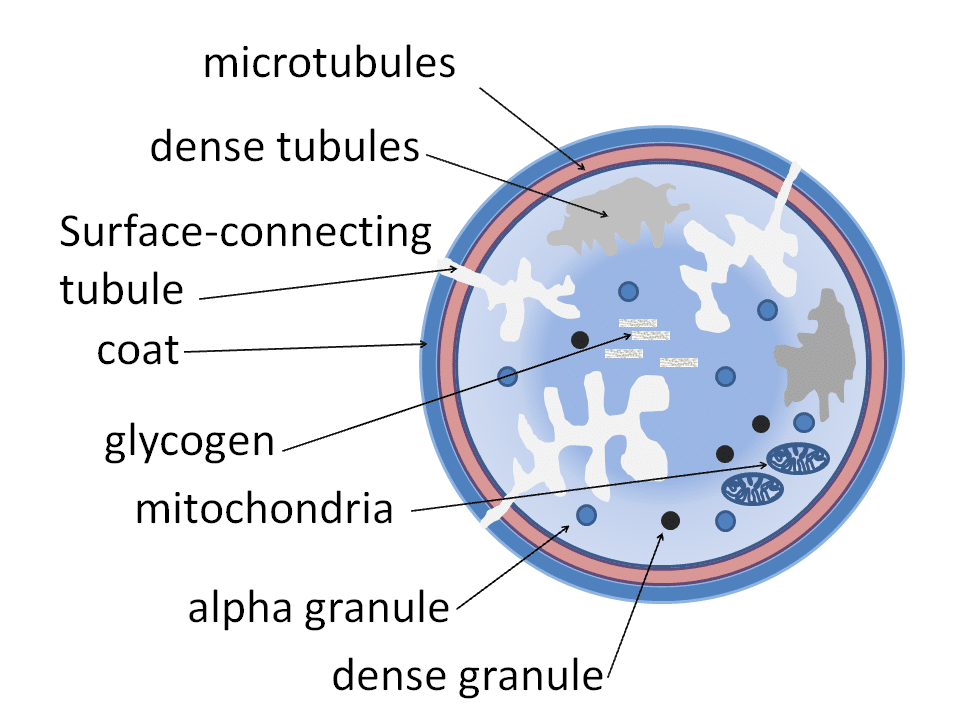
Platelets play an important role in the formation of blood clots. They are commonly described as cellular fragments – they are not true cells as they do not contain a nucleus or carry nuclear DNA, although they do contain mitochondria.
Platelets have a life span of 7-10 days. The normal platelet count is 150-400 x 109/L. Although they are primarily found in the bloodstream, up to 30% of platelets are transiently sequestered within the spleen, ready for rapid mobilisation. Their development from megakaryocytes is stimulated by thrombopoietin.
In this article, we will consider the structure, function and clinical relevance of platelets.
Structure
Platelets originate from megakaryocytes, which are the largest progenitor cells of the bone marrow. Megakaryocytes originate from the common myeloid progenitor.
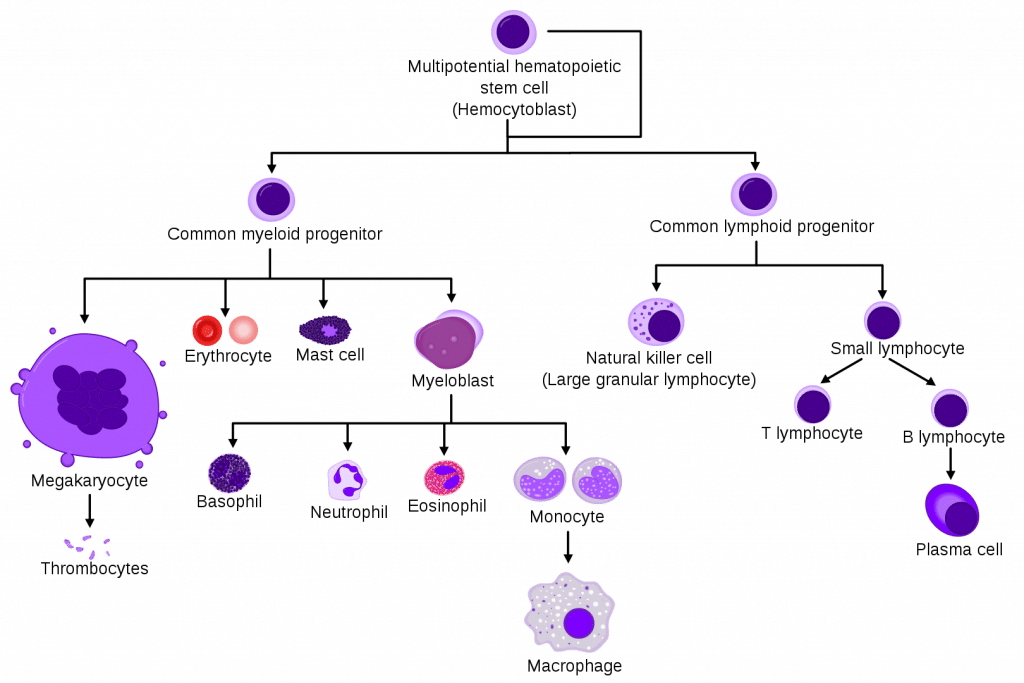
Fig 1 – The cell line of haematopoiesis showing the position of megakaryocytes
They contain two types of granules, namely alpha-granules and dense granules. Alpha-granules contain proteins of high molecular weight, including von Willebrand Factor (vWF), factor V and fibrinogen. Conversely, dense granules contain low molecular weight molecules such as ATP, ADP, serotonin, and calcium ions.
Platelets have abundant surface receptors, classified into agonist and adhesion receptors. Agonist receptors recognise stimulatory molecules. These include collagen, thrombin, and ADP amongst others.
Adhesion receptors promote the adhesion of platelets to other platelets, the vessel wall or leucocytes, depending on the receptor stimulated. Examples include the glycoprotein IIb-IIIa receptor, which is targeted by antiplatelets such as tirafiban.
Function
The key role of platelets is their participation in haemostasis through the formation of blood clots at the site of bleeding.
There are three main stages in the formation of a blood clot: adhesion, activation and aggregation.
Adhesion
Injury to the blood vessel wall exposes its underlying endothelium and collagen fibres. Exposed collagen fibres bind vWF released from the damaged endothelium, which in turn binds to vWF receptors on platelets to promote adhesion. The exposed collagen itself also promotes platelet binding.
Furthermore, the exposed collagen triggers the clotting cascade which, through the utilisation of tissue factors circulating in the blood, generates thrombin, also known as factor IIa.
Thrombin then converts the soluble fibrinogen (factor I) into its insoluble form, fibrin, to create a dense network of fibrin fibres. This fibrin net enmeshes circulating platelets to form a platelet plug and make a stable clot.
Factor VIII plays an important role in the intrinsic pathway, however, it is not very stable and rapidly broken down. Von Willebrand’s factor (or vWF) stabilises factor VIII by binding to it, thus preventing degradation.
Activation
When a platelet binds to collagen, the glycoprotein IIb/IIIa pathway is activated – a complex system controlled by G-protein coupled receptors (GPCRs). The result is the secretion of ADP and thromboxane A2 which subsequently activate other platelets.
Platelet activation results in a morphological change on the membrane surface of the platelet, increasing the surface area and preparing it for aggregation.
Aggregation
Once activated, platelets express the GPIIb/IIIa receptor which can then bind with vWF or fibrinogen. Fibrinogen facilitates the formation of crosslinks between platelets, aiding platelet aggregation to form a platelet plug.
Fibrinolysis
The production of the platelet plug is an example of positive feedback and thus it is necessary to have measures in place to prevent excessive and inappropriate clot formation. Produced in the liver, plasminogen is activated to form plasmin by factors XIa and XIIa. Plasmin breaks down fibrin into fibrin-degradation products – also known as D-dimers.
Therefore, it can be useful to measure the D-dimer levels in a patient where the likelihood of a deep vein thrombosis (DVT) or pulmonary embolism (PE) is low, as a negative D-dimer excludes DVT/PE. D-dimers are non-specific to DVT/PE and can be elevated in pregnancy, after surgery or trauma, to name a few.
Clopidogrel is an antiplatelet often used for secondary prevention of stroke or myocardial infarction. Its primary mechanism of action is the prevention of clot formation, through antagonism of the agonistic ADP receptor. Aspirin irreversibly inhibits cyclo-oxygenase and blocks the production of thromboxane, preventing platelet activation and further aggregation. It can be used in the management of acute coronary syndromes (unstable angina, non-ST elevation myocardial infarction and ST elevation myocardial infarction) and in pregnant women with a moderate or high risk of pre-eclampsia. This is an autosomal dominant disease characterized by very low levels of vWF, resulting in reduced platelet adhesion and reduced factor VIII activity. As they do not have an adequate clotting system, these patients are predisposed to bleeding. Therefore, patients present with frequent, long-lasting epistaxis, large and easy bruising, bleeding gums, and, in women, menorrhagia and post-partum haemorrhage. It is managed by administering desmopressin (to release intrinsic stores of vWF and factor VIII) and tranexamic acid (an antifibrinolytic) to help achieve haemostasis. Thrombocythemia is where the serum platelet count increases, sometimes over 1000×109/L. This can be primary (of unknown cause) or secondary, as a result of bleeding, infection, chronic inflammation from rheumatoid arthritis/IBD, trauma/surgery or hyposplenism. This puts the patient at an increased risk of thrombosis, both arterial and venous: Patients are also at an increased risk of bleeding as, although there is an increased number of platelets, they are abnormal in function. Patients with a platelet count of less than 1000×109/L can be treated solely with aspirin, whilst counts over 1000×109/L may require alternative, more powerful medication.Clinical Relevance – Antiplatelets
Clinical Relevance – Von Willebrand’s Disease
Clinical Relevance – Thrombocythemia