Antibodies, or immunoglobulins, are Y-shaped glycoproteins produced by differentiated B-cells called plasma cells. They are present in bodily fluids, secretions and on the surface of B-cells. Antibodies recognise and bind to unique epitopes, which are molecular structures on the surface of their cognate antigens.
In this article, we will consider antibody structure, function, classes and clinical relevance.
Structure
Heavy and light chains
Antibody molecules consist of two identical heavy chains and two identical light chains, which consequently give the antibody two antigen-binding sites. Disulphide bonds bind the heavy chains to each other and to the light chains (Figure 1). In addition, the heavy and light chains consist of several amino-acid sequences; each corresponding to a protein domain. Proteins domains are the functional units of the antibody and correspond to a discrete, folded region of protein structure. They are, therefore, relevant in antibody engineering (see ‘monoclonal antibodies’ below). Each light chain has two domains (one variable and one constant), and each heavy chain has four (one variable and three constant).
There are five heavy chain types: μ (Mu), γ (Gamma), α (Alpha), ε (Epsilon) and δ (Delta), which classify IgM, IgG, IgA, IgE and IgD respectively.
There are two light chain types: κ (kappa) and λ (lambda). Each antibody can have either two κ or two λ chains but not one of each. The ratio of κ and λ is 2:1. However, there are no functional differences between the types.
Fc and Fab regions
Each antibody contains two variable regions and one constant region.
The Fab regions (fragment antigen binding) contain the variable domains of the light and heavy chains. The variable domains make up the variable regions of the antibody which give the antibody its antigen specificity. Therefore, these regions differ between antibodies. Each Fab region also contains two constant domains; one from the heavy chain component and one from the light chain component.
The Fc region (fragment crystallisable) consists of the remaining constant domains from the two heavy chains. The Fc region interacts with different immune cells and mediates various functions. For example, opsonisation (see below).
The constant region involves the constant domains from both the Fab and Fc parts. The heavy chain constant domains determine antibody class and are the same for all antibodies of the same class.
IgA and IgG antibodies also have hinge regions, which are flexible amino-acid chains in the central part of the heavy chains.
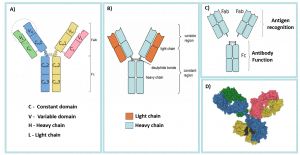
Fig 1 – A) the constant and variable domains of the heavy and light chains; B) Antibody Light chains and heavy chains; C) Fab and Fc regions and functions; D) 3D illustration of antibody structure.
Classification
Antibodies are classified according to heavy chain type, which is encoded by a gene on chromosome 14. The different classes are IgG, IgA, IgM, IgD and IgE; in descending order of abundance in serum.
IgG
IgG is the most abundant antibody class. It is present on the surface of mature B-cells and in serum. There are four subclasses: IgG1, IgG2, IgG3 and IgG4; in order of serum concentration. IgG is the only antibody to cross the placenta and consequently, it transfers passive immunity from mother to foetus. Newborns, therefore, have high IgG concentrations in the first 3-6 months of life.
IgA
IgA is the most prevalent antibody in secretions, such as saliva and mucous. There are two subclasses, IgA1 and IgA2. IgA forms a dimer, where a joining chain connects 2 Y-shaped molecules, giving it four antigen-binding sites in total. IgA antibodies are resistant to enzymatic digestion and act principally as neutralising antibodies. Breast milk and colostrum have high levels of IgA which coat the aerodigestive tracts; protecting against infections in breast-fed babies.
In adults, IgA forms a barrier layer at mucosal surfaces to prevent pathogenic invasion. Plasma cells in the lamina propria produce excessive amounts of polymeric IgA which then moves by endocytosis through the epithelial layer to be secreted at the luminal side. IgA neutralises pathogens and hinders their attachment to epithelial receptors by binding to their ligands on pathogens or toxins. IgA molecules can also cross-link polyvalent antigens or pathogens, forming antigen-antibody complexes which are then trapped in the mucus layer and cleared through peristalsis.
IgM
IgM antibodies are expressed on the surface of B-cells as monomers but secreted as pentameters. A pentameter has five antibodies connected by a joining chain, with ten antigen-binding sites in total. It is the first immunoglobulin produced during foetal development and the first to be produced by B-cells against a new infection. IgM has high avidity, meaning the antibody-antigen complex is strong, but low affinity, so the strength of a single epitope-antibody interaction is weak.
IgD
IgD is present on the surface of B-cells. It has a role in B-cell and antibody production. All naive B cells express IgD and IgM.
IgE
IgE is mainly found on mast cells but is also present at low levels in the blood and extracellular fluid. It is associated with allergy, particularly type I hypersensitivity reactions, including atopic disease (e.g. asthma and dermatitis) and anaphylaxis. It triggers histamine release from mast cells and basophils. IgE is also part of the body’s response to parasitic infections.
Function
The Fc region binds different immune cell receptors (e.g. on phagocytes) and mediates various effector functions.
Opsonisation
Antibodies (mainly IgG1 and IgG3) can act as opsonins by binding to the pathogen, which allows better recognition by phagocytes. Phagocytes then bind to the antibodies via their Fc receptors and initiate phagocytosis.
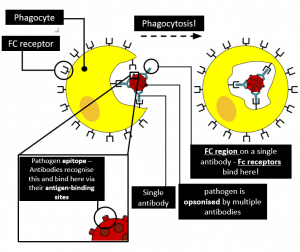
Fig 3 – Pathogen opsonised by multiple antibodies; Phagocytes bind to these antibodies via their Fc receptors and initiate phagocytosis.
Neutralisation
Antibodies can prevent pathogens from accessing cells by blocking different parts of the bacterial or viral cell surface. Consequently, this neutralises certain viruses and bacterial toxins. Neutralising antibodies must have high affinity to be effective; IgG and IgA antibodies have the greatest effect.
Complement Activation
The classical complement pathway can be activated by IgM or IgG antibodies when they bind to microbial surfaces. This releases C3b, which acts as an opsonin, and other complement components which make up the membrane attack complex. MAC punches holes in the pathogen plasma membrane which leads to cell lysis and death.
Immune Complexes
The binding of multiple antigens and antibodies together can form immune complexes. Complex formation limits the antigens’ diffusing ability, making it easier for phagocytes to find and ingest pathogens through phagocytosis.
Antibody-Dependent Cell-Mediated Cytotoxicity
Antibodies bind and opsonise target cells. Natural killer cells then recognise the Fc portion of the antibody and release cytotoxic granules (perforin and granzymes) into the target cell which triggers apoptosis. They also release interferons, which attract phagocytes.
Clinical Relevance – Autoantibodies
Autoantibodies are antibodies that react against the body’s own antigens. They arise when the immune system cannot distinguish between self and non-self. While healthy people can have autoantibodies, they can indicate or lead to autoimmune disease in some individuals. For example, antibodies against thyroid peroxidase (TPO antibodies) in Hashimoto’s disease. Below are other common examples of autoimmune diseases.
| Autoantibody | Antibody target | Disease |
| Rheumatoid factor (RF) | Fc portion of IgG | Rheumatoid arthritis |
| Anti-thyrotropin receptor antibodies (TRAbs) | TSH receptor of thyroid | Graves’ disease |
| Anti-tissue transglutaminase antibodies (anti-tTG) | Tissue transglutaminase enzyme | Coeliac disease |
Clinical Relevance – Monoclonal Antibodies
Monoclonal antibodies are man-made molecules designed to act as antibodies. In cancer treatment, monoclonal antibodies can bind to cancer-specific antigens and subsequently induce an immune response against cancer cells. For example, trastuzumab (aka Herceptin) is used for HER2 receptor-positive breast cancer. Monoclonal antibodies can also treat autoimmune diseases. For instance, infliximab is an effective treatment for inflammatory bowel disease and rheumatoid arthritis. It works by binding and neutralizing TNF-α.