External balance of potassium is achieved through balancing dietary intake, intracellular and extracellular potassium levels and excretion by kidneys.
Extracellular potassium concentration is usually maintained within 3.5-5 mmol/L. This narrow window of control is critically important as the difference between the intracellular and extracellular potassium affects electrically excitable muscle and nerve cells due to its effect on the resting membrane potential.
Intracellular potassium levels, which are maintained within 120-150mmol/L, are important for enzyme function, cell division and growth. It also contributes to acid-base and cell volume regulation.
This article focuses on the external balance of potassium within the body.
Renal Handling of Potassium
Potassium levels are controlled by regulating its secretion and reabsorption. This is done by the kidneys to match potassium intake and maintain an external balance of potassium
Potassium is freely filtered at the glomerulus and passes through to the proximal convoluted tubule (PCT) and loop of Henle, where most of it is reabsorbed. There is some reabsorption in the distal convoluted tubule and collecting duct, but potassium secretion also occurs at these sites.
Reabsorption of Potassium
The freely filtered potassium is then passed through the kidney tubules. Two-thirds of the filtered K+ is reabsorbed in the PCT and approximately 20% is reabsorbed in the thick ascending limb of the Loop of Henle. This means a very small proportion of K+ reaches the distal nephron.
Proximal Convoluted Tubule
K+ reabsorption occurs passively within the PCT and about two-thirds is reabsorbed here. It occurs via a paracellular mechanism and is directly proportional to water and Na+ movement.
The Na+-K+-ATPase causes Na+ to move out of the proximal tubule cell and drives K+ into the cell. The extrusion of Na+ creates an osmotic gradient and an electrochemical gradient. Water moves out of the PCT down the osmotic gradient and Cl– moves down the electrochemical gradient. K+ is reabsorbed and follows Cl– into the bloodstream.
Thick Ascending Limb of Loop of Henle
In this section of the nephron, roughly 20% of K+ is reabsorbed through transcellular and paracellular pathways.
Transcellular Mechanism
Na+-K+-ATPase on the basolateral membrane pumps Na+ out into the bloodstream and pumps K+ into the thick ascending limb which keeps the sodium concentration in the cell low.
This creates a gradient for the sodium-potassium-chloride (NKCC2) cotransporter on the apical membrane. NKCC2 pumps Na+, K+ and 2 Cl– into the cell from the lumen. Intracellular K+ can enter the bloodstream through K+-Cl– symporter or through the K+ uniporter.
Paracellular Mechanism
Movement of K+ through apical renal outer medullary K+ (ROMK) channels. This leads to a positive voltage in the lumen which provides a driving force for passive reabsorption of K+.
Distal Convoluted Tubule and Cortical Collecting Duct
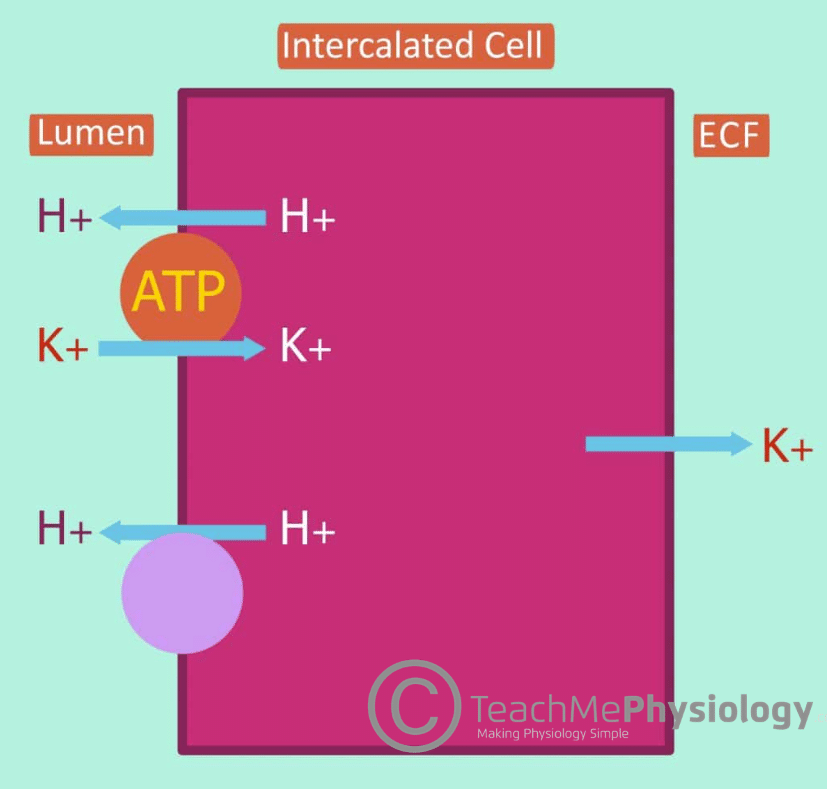
Around 10% of filtered potassium is reabsorbed here when the body is attempting to preserve potassium. It occurs via the transcellular pathway and is mediated by alpha and beta-intercalated cells. Structurally, the initial collecting tubule and cortical collecting duct are both composed of 70% principal cells (secretion of K+) and 30% intercalated cells (reabsorption of K+).
In this section, we are considering the intercalated cells in the reabsorption of K+.
There are two steps in the reabsorption of potassium here:
- The apical H+-K+-ATPase mediates the movement of H+ into the lumen, driving K+ into the intercalated cell.
2. Then, the basolateral K+ channel allows the K+ inside the intercalated cell to leak out into the bloodstream.
In potassium depletion, the number of H+-K+-ATPase pumps increases significantly in order to reabsorb as much K+ as possible. However, reabsorbing K+ drives H+ secretion into the lumen. This leads to hypokalaemic alkalosis.
Secretion of Potassium
Potassium secretion occurs mainly in the late distal collecting tubule (DCT) and the collecting duct (CD). The purpose of secretion is to control the serum potassium levels in the long term. The rate of secretion is variable and can be increased or decreased due to several factors which will be considered later in the article.
With a normal or high K+ diet, the substantial secretion varies between 15-20%. However, with a low K+ diet or depletion, there is very little secretion.
Distal Convoluted Tubule and Collecting Duct
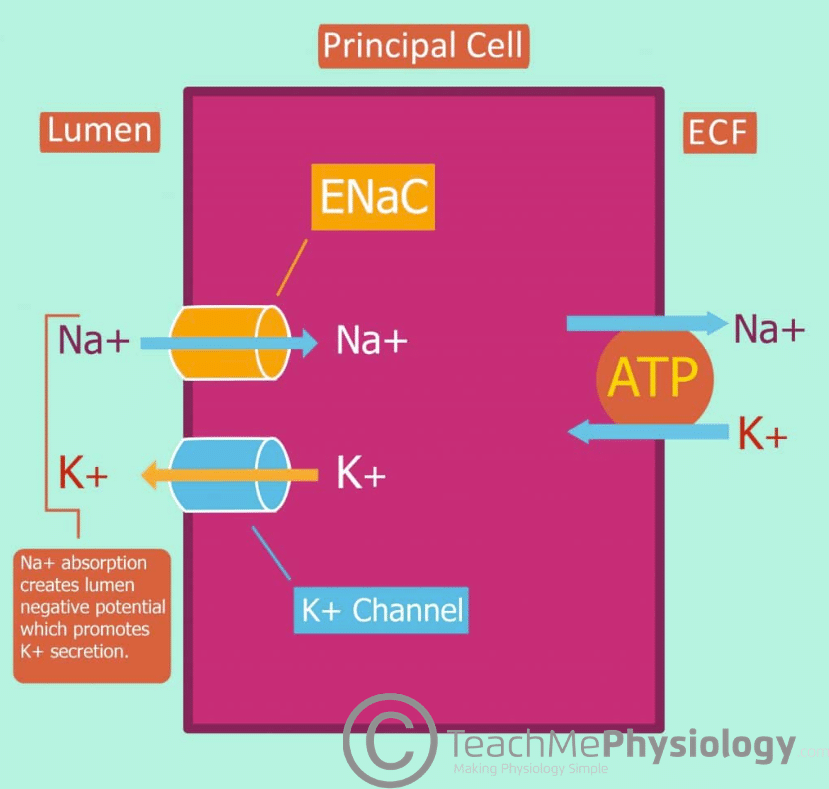
Potassium secretion in the late DCT and CD is mediated via principal cells and the rate can be varied depending on need.
The principal cells of the late DCT and collecting duct contain ENaC on the apical membrane and Na+-K+-ATPase on the basolateral membrane. The activity of Na+-K+-ATPase results in Na+ moving out into the blood from the principal cell and in turn, drives K+ into the principal cell from the bloodstream. This leads to a decrease in intracellular Na+ concentration and an accumulation of intracellular K+.
The high intracellular K+ in comparison to the luminal K+ concentration creates a chemical gradient which is ideal for potassium secretion from the principal cell into the lumen.
Due to the action of Na+-K+-ATPase, the low intracellular [Na+] allows for a concentration gradient between the lumen and principal cell. Na+ moves from the lumen into the cell down the concentration gradient through ENaC.
This creates a favourable electrochemical gradient which allows for K+ secretion via K+ channels on the apical membrane.
Factors Affecting Secretion of Potassium
Tubular Factors
- High ECF [K+] – This stimulates the Na+-K+-ATPases, leading to increased permeability of K+ channels on the apical membrane. This results in increased secretion of K+ into the lumen.
- Aldosterone – This stimulates the Na+-K+-ATPases in the basolateral membrane. This stimulates K+ channels and ENaCs in the apical membrane, leading to increased K+ secretion.
- Acidosis – This leads to increased H+ secretion into the lumen to correct acidosis
- Due to H+-K+-ATPase, when H+ is secreted into the lumen, K+ is driven back into the cell, leading to decreased K+ secretion.
- Alkalosis – The kidneys try to decrease the secretion of H+, increasing the secretion of K+ in turn
- Stimulates Na+-K+-ATPase, leading to increased K+ channel permeability.
Luminal Factors
High luminal flow – The increased flow rate washes away luminal K+, meaning there is a constant concentration gradient available. This leads to increased K+ secretion.
- This increased luminal flow also increases Na+ delivery to the tubule cells which stimulates Na+ uptake through ENaC. This leaves the lumen in a negative potential, encouraging K+ to be secreted through the apical K+ channel.
Clinical Relevance – Hyperkalaemia
Hyperkalaemia is defined as extracellular [K+] > 5mmol/L. It can be caused by decreased excretion, redistribution from intracellular to extracellular or increased K+ intake. Decreased excretion can be a result of renal failure, or Addison’s disease, which leads to increased K+ reuptake.
Redistribution is mainly caused by metabolic acidosis (e.g. diabetic ketoacidosis). This is because H+ is reabsorbed into the cells to try and decrease the pH, so K+ is excreted to maintain electrical equilibrium. Increased K+ intake is usually due to a high intake of salt substitutes or transfusion of stored blood.
Clinical Features
- Kussmaul respirations (rapid, deep breaths) – usually due to an underlying metabolic acidosis trying to be compensated by removing CO2 faster
- Muscle weakness – the changes in membrane potential affect the muscle’s ability to contract, causing weakness.
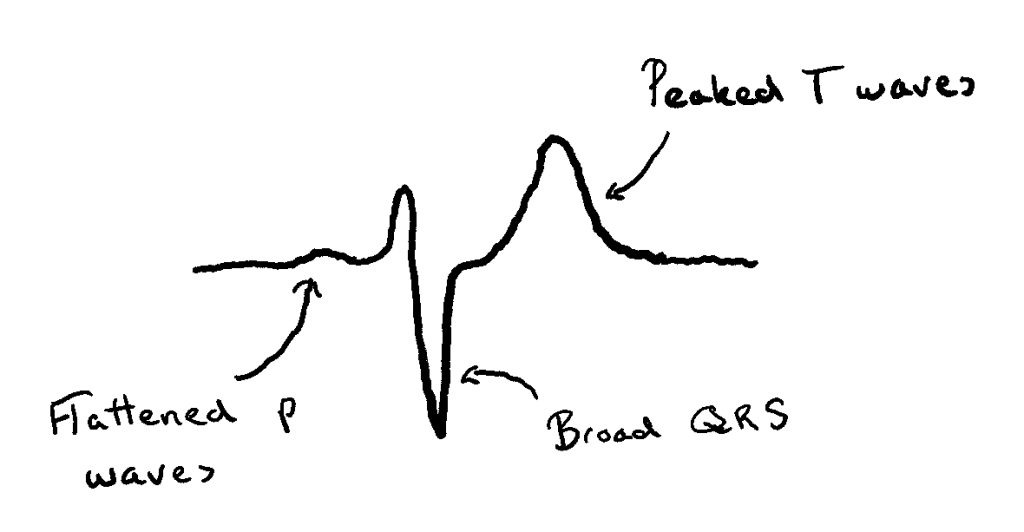
- Cardiac function is also affected due to the chemical gradient of K+ being decreased. This, in turn, makes the cardiac cells less likely to depolarize. ECG changes associated with this include:
- Tented (or peaked) T waves
- Flattened/absent P waves
- Increased PR segment length
- Abnormal QRS complexes
Treatment
Hyperkalaemia is a medical emergency. Most symptoms of hyperkalaemia are only visible once [K+] reaches a level high enough to cause cardiac arrest.
Hyperkalaemia should be treated even before a definitive cause is found. Calcium gluconate is given as an IV bolus, which alters the threshold potential and therefore reduces the risk of arrhythmia.
Nebulised B2 agonists e.g. salbutamol and/or IV insulin-dextrose are also given to encourage K+ reuptake into cells.
Clinical Relevance – Hypokalaemia
Hypokalaemia is when there is not enough K+ in the ECF outside cells and in the blood. To learn more about it, click here.